Generic Drugs
Introduction
When a customer walks into your pharmacy to purchase medications, you will offer them 2 options: original brand or generic brand.
Although generic brand is much cheaper, there are many patients who have total loyalty to proprietary brand, and point that generic brand is the cheaper brand, and hence, an inferior brand with more adverse effects profile.
- But, is this a fact?
NOTE: Do you know that?
- Sandoz is a division of the Novartis Group, focusing on generic pharmaceutical and biosimilars.
- Winthrop is a Sanofi Company which aims to make it possible for patients to receive high-quality Sanofi generic pharmaceutical products at an affordable price.
Generic Drugs
Typically, a new drug will have a patent protection of typically 20 years. Once the patent expires, a pharmacologically equivalent version may be sold as a "generic" version of the brand name drug.
- The generic drug manufacturer must ensure that the drug they are producing contains the same active ingredient(s) as the brand-name product, in the same dosage form, at the same dose or concentration and for the same route of administration.
- However, it may differ in characteristics such as colour, shape, taste, inactive ingredients, preservatives and packaging.
- Because of these differences, the generic drug manufacturers are required to submit additional paperwork to the authority to prove that their product is manufactured in accordance with good manufacturing practices, and is as pure and stable as the brand-name product.
The World Health Organization considers two formulation bioequivalent if the 90% confidence interval for the ratio multisource (generic) product/comparator lie within 80.00-125.00% acceptance range for AUC0-t and Cmax.
- If the active pharmaceutical ingredient is determined to posses a narrow therapeutic index, the bioequivalence acceptance range may need to be restricted to 90.00-111.11%.
FDA Orange Book
Theoretically, any generic drug that is bioequivalent to its brand-name counterpart may be interchanged with it. There are cases where a generic product may not be appropriate:
- Small differences in the amount of drug in the bloodstream can make a very large difference in the drug’s effectiveness, such as warfarin and phenytoin.
- Generic product contains an inactive ingredient that the patient is allergic to.
At US, Approved Drug Products With Therapeutic Equivalence Evaluations, also known as "the orange book" is published by the FDA each year and updated periodically to provide guidance about which drugs are interchangeable. The two basic categories into which multisource drugs have been placed are indicated by the first letter of the relevant therapeutic equivalence code as follows:
- A - Drug products that FDA considers to be therapeutically equivalent to other pharmaceutically equivalent products, i.e., drug products for which:
- there are no known or suspected bioequivalence problems. These are designated AA, AN, AO, AP, or AT, depending on the dosage form; or
- actual or potential bioequivalence problems have been resolved with adequate in vivo and/or in vitro evidence supporting bioequivalence. These are designated AB.
- B - Drug products that FDA at this time, considers not to be therapeutically equivalent to other pharmaceutically equivalent products, i.e.,
- drug products for which actual or potential bioequivalence problems have not been resolved by adequate evidence of bioequivalence. Often the problem is with specific dosage forms rather than with the active ingredients. These are designated BC, BD, BE, BN, BP, BR, BS, BT, BX, or B*.
Why Generic Drugs are much Cheaper?
In university, we all have been educated to believe that generic brand medications work just as comparable to original brands, but at a much competitive price.
- It is a similar concept to buying a handbag from the market.
- You can buy an expensive branded or a cheaper no-branded one, but both of them will perform the exact same function.
The reason that generic drugs tend to cost less is because they do not have to repeat animal and clinical (human) studies that were required to demonstrate safety and effectiveness.
- In reality, the approval of multiple applications of generic drugs also creates competition in the marketplace and resulting in lower prices.
- To illustrate, you may have a one-month supply of atorvastatin at a third of the price of Lipitor.
Authorized Generics
Authorized generics are manufactured by the innovator company and are the same as the brand name drug in all aspects, with the exception of not using the brand name on the label.
- FDA publishes a list of reported authorized generics and updates that list quarterly.
- An example will be Axiago (Esomeprazole) manufactured by AstraZenca.
The approach of authorized seems to increase the access of medicine at a more affordable price.
- However, it is actually another strategy for innovator to protect their sales once their patents expire, and meanwhile it also erodes incentive to make generic drugs from other companies.
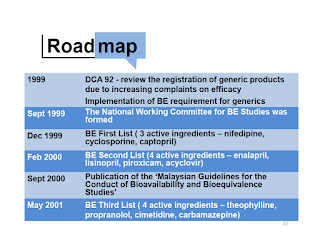
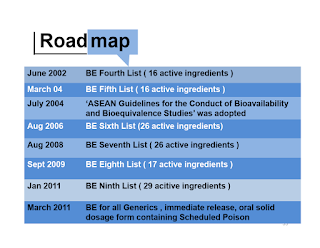
Implementation of Bioequivalence Studies for Generics Medicines in Malaysia
In Drug Control Authority 92nd Meeting in 1999, it is decided to include bioequivalence (BE) studies as a requirement for generics mediations (oral immediate release solid dosage form) due to
- Increased availability of generics
- Increased complaints on efficacy
Effective from 1st January 2012, bioequivalence (BE) studies are required for all generic medicines which are in the form of immediate release, oral solid dosage and the BE studies shall be conducted at BE center accredited by NPCB in order to support the registration of generic medicine in Malaysia.
- Failure to fulfil BE requirements will result in cancellation or suspension of registered product and rejection of application for registration by the DCA.
- At the moment, BE studies are not a pre-requisite registration of generic medications for dosage forms other than oral, solid dosage forms.
Since March 2015, Malaysia has fully adopted ASEAN Guideline for the Conduct of Bioequivalence Studies.
Concerns on Generic Brands Quality
In reality, is the generic medication story as rosy as what we believed?
Although pharmacist is a self-proclaimed drug expert, many of us may not know the news of generic drug manufacturer Ranbaxy pleads guilty and agrees to pay $500 million to resolve false claims allegations, cGMP violations and false statements to the FDA in 2013.
- Ranbaxy acknowledged that there is an incomplete testing records and inadequate stability program, as well as significant cGMP deviations in the manufacture of certain active pharmaceutical ingredients and finished products.
- "Stability" refers to how the quality of a drug varies with time under the influence of a variety of factors, such as temperature, humidity, and light. Such testing is used to determine appropriate storage conditions and expiration dates for the drug, as well as to detect any impurities in the drug.
- This has certainly raised the question of could there be other generic drug makers falsifying records, covering up manufacturing problems and attempting to hide poor test results.
2019 is a year haunted with generic brand medications contaminated with N-nitrosodimethylamine (NDMA) that exceeded internationally acceptable limits.
- It first started with product recall of losartan in the mid of the year, followed with halted worldwide distribution of generic ranitidine.
- In December 2019, Singapore Health Sciences Authority recalls 3 versions of metformin out of 46 locally marketed metformin.
- With this issue, consumers (patients) are terrified at the risk of exposing themselves with carcinogens when using generic medications. At outpatient pharmacy setting, some patients even ask if their medication is safe even when they are just on amlodipine as their sole medication for antihypertensive management.
This brings us to the next question: should our health being compromised with a cheaper price tag?
- I hope this is not the case.
- What could have led to this predicament is that companies manufacturing generic drugs have to only show patients will absorb them at the same rate as the name-brand medications they mimic.
- The regulatory system for generics is basically built on trust, specifically trust in manufacturers.
In years to come, a better regulatory system on generic medications should be enforced to ensure the generic medications are of high quality yet at an affordable price.
- However, this goal may be challenging to achieve, as profit maximization remains a primary driver in modern capitalism.
Summary
If you ask me, should we purchase more affordable generic medications or proprietary brand medications with demonstrated effectiveness?
- When cost is a primary concern, particularly for long-term medication use, opting for cost-effective generic alternatives can be a wise option.
- However, it is important to remember that cost should not be the sole deciding factor.
- In situations where the effectiveness of generic medications is suboptimal or where they cause unacceptable adverse reactions, switching to proprietary brand medications may be necessary.
External Links
- FDA - Generic Drug Facts
- Implementation of Bioequivalence Studies for Generic Medicines in Malaysia, 2011
- Generic Drug Manufacturer Ranbaxy Pleads Guilty and Agrees to Pay $500 Million to Resolve False Claims Allegations, cGMP Violations and False Statements to the FDA, 2013
- Carcinogens Have Infiltrated the Generic Drug Supply in the U.S., 2019
- Generic Zantac Distribution Halted Worldwide, 2019
- HSA recalls 3 versions of diabetes drug metformin amid global testing for carcinogen, 2019
- FDA lambasts Kilitch for unsanitary manufacturing conditions and issues warning letter to Natco, too, 2024

Comments
Post a Comment