Research
Introduction
Participating in research is one of the requirements for provisional registered pharmacist training at government hospital.
- However, many of us may have little ideas on how to carry out research.
- Hence, I would like to write a brief introduction on it.
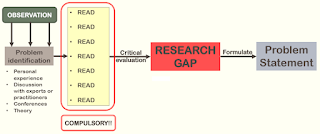
Identifying Research Gap
No doubt, brainstorming to think of a good problem to address is a very long process. After identifying the problem, it should be followed by a literature review (which requires lots of readings!!!). Some of the databases that you may use should include:
From literature review, we will have a better understanding of the topic and be able to identify if there is any knowledge gap or discrepancies in results.
- Only then, you will decide if there is still a need to proceed with your current research or how will you design your study.
Remember, fundamentally, the purpose of a research is to find an answer that is not readily available yet.
- For example, we may want to know if we are having a heart attack, should we chew or swallow aspirin tablet to have the fastest onset of action or it does not matter.
Always ask yourself, why do you want to know the answer of your study, how do you plan to measure the result and how will the answer affect your future recommendation (or what is the implication).
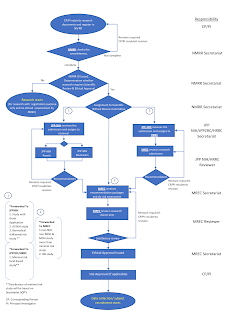
Flow Chart of Investigator Initiated Research
All research undertaken by MOH personnel or conducted in MOH institutions or using MOH data/ patient/ sample/ personnel as subject or funded by MOH Research Grant MUST be registered with NMRR prior to data collection and/or subject recruitment.
- Unique NMRR registration ID will be assigned for each research.
- All investigators involved in the research are required to have a registered account in NMRR to be listed as an investigator. The investigator is required to submit his or her curriculum vitae.
- Principal investigator (PI) and at least 1 sub-investigator must sign an Investigator Agreement and obtain approval from his or her HOD and institutional/organisational director by using the IA-HOD-IA Form (Investigator's agreement, Head of Departments and Institutional approval).
- For collaborative research with any external organisation or entity outside of MOH, a Memorandum of Understanding (MoU) or Memorandum of Agreement (MoA) and Research Agreement (RA) between the related MOH division, institution of facility and the external party must be obtained.
- Good Clinical Practice (GCP) certificate and valid professional indemnity certificate are needed for interventional research.
Research protocol that has been uploaded onto the NMRR website will be forwarded to the respective authorities for review and rating recommendation for Medical Research Ethics Committee (MREC) [or known as "Jawatankuasa Etika dan Penyelidikan Perubatan" (JEPP)] approval.
- Only after obtained ethical approval, recruitment of subjects and/or data collection can start.
- Sites without a signed IA-HOD-IA form should also obtain approval to conduct research at each site via the Site Approval Form.
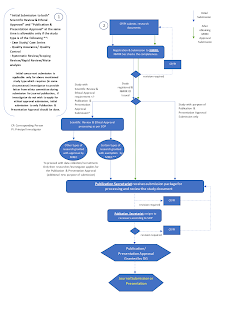
During the conduct of research
- Any subsequent changes or additions to research have to be submitted, reviewed and approved by MREC before incorporation to research.
- Applications for renewal of ethical approval should be made on a yearly basis and should be submitted prior to the expiry of the ethical approval.
- The Closure/Suspension/Termination of research should be notified to MREC.
Finally, all dissemination of scientific outputs such as abstracts for oral or poster presentation, research reports, journal articles, conference proceedings undertaken by MOH personnel OR utilising data of MOH patient/ sample/ personnel as subject OR funded by a MOH research grant shall require review and subsequent approval by the Director-General of Health prior to publication and presentation.
Research Design
Just before we start to collect data, we should plan our study design well. If not, we may need to restart the data collection again just because of a missing data. There are few key factors that we should consider.
Study type
- Although randomised controlled trial is the gold standard of scientific evidence, each type of research study (e.g. cross-sectional study, case study, case-control, cohort and clinical trial) has its own strengths and weaknesses. In other words, you should choose the most appropriate study type which answers your problem.
- You should also determine the time perspective of your data collection
- Retrospective - from available past records
- Cross-sectional - at one point in time
- Prospective - collecting new data over time
Ethical considerations
- All medical research in Malaysia must be registered with the NMRR and requires ethical review.
- Institutional approval is also required before data collection start.
- DO NOT HARM the respondents!!!
Variables
- We will need to identify the variables that we would like to collect, e.g. outcome and confounding factors that may contribute to the outcome of interest.
- Determine the type of variables
- Continuous measured/scale, e.g. blood pressure in mmHg
- Ordinal, e.g. Likert scale - Strongly disagree to strongly agree
- Categorical/nominal, e.g. gender, yes/no
- What are the inclusion and exclusion criteria in this study? (and why?)
- Sampling size - A sample size calculation should be performed. This is so that we recruit enough people for us to answer correctly but not to recruit excessive participants. May discuss with statistician for details.
- Sampling method - probability sampling (e.g. random sampling) or non-probability sampling (e.g. convenience)
- Interview
- Self-administered questionnaire
- Recorded sources
- Observation
- Focus group discussion
- One of the most common statistical packages that we use is SPSS. If you need some tutorials on it, you may look it up here. Some may use R programming or jamovi.
- Always remember that statistical package can always run the statistical test for you, but it does now differentiate if you are using the correct test or not. So, if any doubt, please consult statistician.
- Descriptive statistics
- Visual methods of describing data include
- Frequency distribution
- Histogram
- Scatterplot
- Boxplot
- Numerical methods
- Arithmetic mean
- Median
- Mode
- Standard deviation
- Range
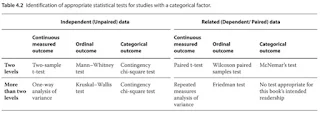
- Choosing the appropriate statistical test
Gantt chart
- Map out the research activities by timeframe so that we able to monitor our progress.
Once you have completed the research proposal, I would say you are half-way done. As you would have expected, you will then start to collect and analyse the data and eventually write up the final report.
Statistics
If you would like to brush up your knowledge on statistics, I personally recommend you to have a read on A Practical Approach to Using Statistics in Health Research.
- It is a concise, nicely written reference. If any doubt, consult a statistician.
External Links
- Medicines Learning Portal - Research
- National Medical Research Register
- NMRR Template Forms
- Garis Panduan Menjalankan Penyelidikan di Program Perkhidmatan Farmasi, Kementerian Kesihatan Malaysia, 2022
- NIH Guidelines for Conducting Research in Ministry of Health (MOH) Institutions & Facilities, 2021
- Writing a manuscript for publication in a peer-reviewed scientific journal: Guidance from the European Society of Clinical Pharmacy, 2024

Comments
Post a Comment