Extemporaneous Preparation with Unknown Stability
Introduction
Recently, you are assigned the task of updating extemporaneous preparation formulae at your hospital.
- Much to your surprise, a significant number of extemporaneous preparations originated in The Pharmaceutical Codex 1994 (or 1973).
- Although you managed to identify the extemporaneous preparation formulae, no stability data was provided.
Today, stability information can be obtained from manufacturer’s labelling information (such as in the package insert), the USP compounding monographs, or peer-reviewed articles and references, such as articles in American Journal of Health-System Pharmacy, the International Journal of Pharmaceutical Compounding.
- However, what should you recommend if stability data is unavailable?
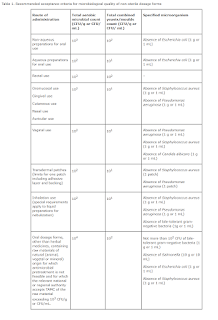
USP General Chapter <795> and <797>
The revision of <795> and <797>, published in November 2022, became official on 1 November 2023, replacing the previous revision of <795> from 2014 and <797> from 2008.
- In general, the use of water activity (aw) aids in assessing the susceptibility of compounded nonsterile preparations (CNSPs) to microbial contimination and the potential for active pharmaceutical ingredient (API) degradation due to hydrolysis.
- When preparing CNSPs, raw materials and equipment contribute a bioburden of the final preparation.
- CNSPs with an aw ≥ 0.6, and a beyond-use date (BUD) within the limits of Table 4, should contain suitable antimicrobial agents to protect against the proliferation of bacteria, yeast, and mold contamination that may be inadvertently introduced anytime during the compounding process or throughout the BUD under appropriate handling and storage conditions.
- Careful consideration should be taken when selecting a preservative to ensure microbiological effectiveness and stability.
- When antimicrobial preservatives are contraindicated in a CNSP, storage of the preparation in a refrigerator is required if such storage does not change the physical or chemical properties of the CNSP (i.e., precipitation).
- The BUDs in Table 4 are the BUD limits for CNSPs in the absence of specific stability information.
- This does not absolve the designated person(s) from performing due diligence to determine if there is existing stability data that would require a shorter BUD.
- When compounding from a USP–NF compounded preparation monograph for the CNSP, the BUD must not exceed the BUD specified in the monograph.
- If the BUD of the CNSP is extended beyond the BUDs in Table 4, an aqueous CNSP must be tested for antimicrobial effectiveness
- Additionally, the BUD of the CNSP must not exceed the shortest remaining expiration date of any of the commercially available starting components.
- For CNSPs prepared from one or more compounded components, the BUD should generally not exceed the shortest BUD of any of the individual compounded components
- More information can be found here.
British Pharmacopoeia
For official preparations of solutions and suspensions, the British Pharmacopoeia employs two definitions.
- “Freshly Prepared” indicates that an extemporaneous preparation must be made within 24 hours of issue for use.
- “Recently Prepared” should be applied to extemporaneous preparations that are likely to deteriorate if stored for more than 4 weeks when maintained at 15-25°C.
Pharmaceutical Compounding and Dispensing, 2010
According to Pharmaceutical Compounding and Dispensing 2010, in the absence of any guide, it is suggested that
- Creams are given a four-week discard date. This is significantly shorter than the suggested discard date for extemporaneously prepared ointments (which is three months) because of the susceptibility of creams to microbial contamination. Diluted creams would normally be given a two-week discard date.
- Ointments and pastes are given a three-month discard date. This is significantly longer than the suggested discard date for extemporaneously prepared creams (which is four weeks) owing to the fact that ointments are less susceptible to microbial contamination. Diluted ointments would normally be given a two-week discard date.
- Gels, which have a higher water content, will attract a shorter discard date. In the absence of any official guidance, it is suggested that gels are given a four-week expiry date.
The International Pharmacopoeia, 2025
According to The International Pharmacopoeia, 2025, the acceptance criteria for non-sterile pharmaceutical products are based upon the total aerobic microbial count (TAMC) and the total combined yeasts/moulds count (TYMC) as table above. When an acceptance criterion for microbiological quality is prescribed, it is interpreted as follows:
- 101 CFU: maximum acceptable count = 20,
- 102 CFU: maximum acceptable count = 200, and so forth
- 103 CFU: maximum acceptable count = 2000, and so forth
In addition to the microorganisms listed in the table, the significance of other microorganisms recovered should be evaluated in terms of:
- the use of the product: hazard varies according to the route of administration (eye, nose, respiratory tract);
- the nature of the product: its ability to support growth, the presence of adequate antimicrobial preservation;
- the method of application;
- the intended recipient - risk may differ for neonates, infants, the debilitated;
- use of immunosuppressive agents, corticosteroids;
- presence of disease, wounds, organ damage.
Australian Pharmaceutical Formulary and Handbook (APF)
According to Australian Pharmaceutical Formulary and Handbook (APF) 25th edition, the following guidance applies to expiry dates for non-sterile compounded medicines:
- The expiry date is 28 days or less from the date the medicine is compounded, unless otherwise specified in the APF or in a reliable stability study. An expiry date of less than 28 days may be more appropriate in some clinical circumstances.
- The expiry date of compounded capsules or powders is 6 months or less from the date the medicine is compounded, provided the ingredients are stable in air and not hygroscopic or deliquescent.
- If the expiry date from a reliable stability study that uses the same formulation, packaging and storage conditions as the compounded medicine differs from the expiry date guidance in the APF (longer or shorter), the expiry date from the stability study should be used.
- The expiry date of a compounded medicine must never be longer than 6 months.
- The expiry date of a compounded medicine must not be later than the expiry date of any ingredient.
Nonetheless, the expiry dates guidance in APF 26th edition is aligned with the United States Pharmacopoeia.








Comments
Post a Comment