Read a Paper
Introduction
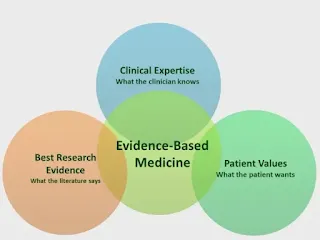
Evidence-based medicine (EBM) is the conscientious, explicit and judicious use of current best evidence in making decisions about the care of individual patients.
- The practice of evidence-based medicine means integrating individual clinical practice with the best available external clinical evidence systematic research.
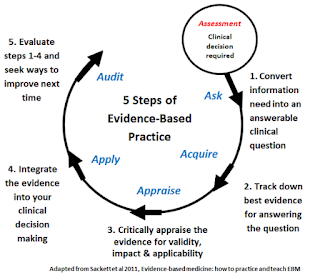
The essential steps include:
- To convert our information needs into answerable questions (i.e. to formulate the problem);
- To track down, with maximum efficiency, the best evidence with which to answer these questions - which may come from the clinical examination, the diagnostic laboratory, the published literature or other sources;
- To appraise the evidence critically to assess its validity and usefulness;
- To implement the results of this appraisal in our clinical practice;
- To evaluate our performance
NOTE: EBM does not mean we justify our approach to a particular problem by citing the results section of a single published study, without critically appraising the study first. A study with methodological flaws should be trashed, although it addressed an important clinical issue with a "statistically significant" result.
Formulate the Problem
A good clinical question includes
- define precisely whom the question is about.
- define which manoeuvre you are considering (e.g. drug, surgery), and if necessary, a comparison manoeuvre (e.g. placebo or current standard therapy)
- define the desired (or undesired) outcome
To do this, you may use the PICO tool (Population, Intervention, Comparison, Outcome).
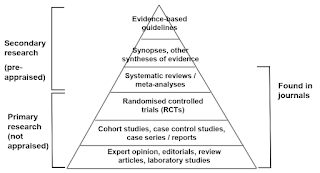
Level of Evidence
The term level of evidence refers to what degree that information can be trusted, based on study design.
Randomised controlled trials (RCTs) are often said to be the gold standard in medical research.
- Up to a point, this is true but only for certain types of clinical questions.
- The questions that best lend themselves to the RCT design relate to interventions, and are mainly concerned with therapy or prevention.
Sample Size Calculation
A trial should be large enough to have a high chance of detecting a worthwhile effect if it exists.
- There are usually restrictions on number of participants (sample size) because of ethical, cost and time considerations.
- Statistician can work out before the trial begins how large the sample size should be in order to have a good chance of detecting a true difference between the intervention and control groups.
- The calculation will also need to include an allowance for participants dropping out.
Critical Appraisal
Critical appraisal is essential to combat information overload and identify research papers that are clinically relevant.
- Assess validity by considering how robust the data are: is the clinical trial scientifically sound?
- Evaluate usefulness by thinking about how far we can generalise the results from trials into routine practice to inform clinical care (i.e. what the results mean for our patients in the real world).
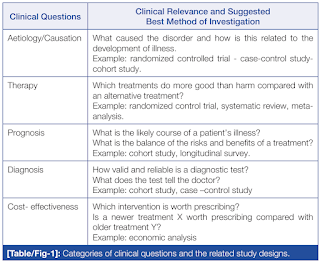
Below is a generalized checklist of 20 questions for journal article written by Robert Will. The first 4 questions should be answered "Yes". If any of the 4 questions are answered "no", then you should return to your search and attempt to find an article that will meet these criteria.
Critical appraisal of…the Introduction
- Does the article attempt to answer the same question as your clinical question?
- Is the article recently published (within 5 years) or is it seminal (i.e. an earlier article but which has strongly influenced later developments)?
- Is the journal peer-reviewed?
- Do the authors present a hypothesis?
Critical appraisal of…the Methods
- Is the study design valid for your question?
- Are both inclusion and exclusion criteria described?
- Is there an attempt to limit bias in the selection of participant groups?
- Are there methodological protocols (i.e. blinding) used to limit other possible bias?
- Do the research methods limit the influence of confounding variables?
- Are the outcome measures valid for the health condition you are researching?
Critical appraisal of…the Results
- Is there a table that describes the subjects’ demographics?
- Are the baseline demographics between groups similar?
- Are the subjects generalizable to your patient?
- Are the statistical tests appropriate for the study design and clinical question?
- Are the results presented within the paper?
- Are the results statistically significant and how large is the difference between groups?
- Is there evidence of significance fishing (i.e. changing statistical tests to ensure significance)?
Critical appraisal of…the Discussion/Conclusion
- Do the authors attempt to contextualise non-significant data in an attempt to portray significance? (e.g. talking about findings which had a trend towards significance as if they were significant).
- Do the authors acknowledge limitations in the article?
- Are there any conflicts of interests noted?
- Critical Appraisal Skills Programme (CASP) Checklist
- Critical Appraisal of Clinical Research, 2017
- Critical appraisal: how to evaluate research for use in clinical practice, 2021
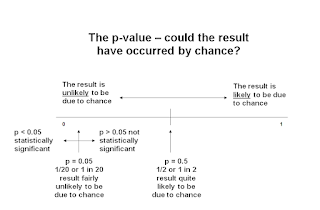
P-Value
A p-value is the probability that a difference will been seen between two interventions in a trial.
- In other words, it is an indication of whether the result occurred by chance.
However, p-values can be overtrusted and misused, because
- It depends on sample size.
- The effect may be small and clinically unimportant, but the p-value can still be significant if the sample size is large.
- On the other hand, an effect can be large, but fail to meet the p<0.05 criterion if the sample size is small.
- The p-values are based only on data from a sample of people.
Summary
An important aspect often overlooked is that the absence of evidence does not, in itself, signify the absence of effectiveness.
- The current preference is to utilize treatments with clear evidence of benefit and to avoid treatments with evidence of ineffectiveness (evidence of absence).
- However, day-to-day practice may encounter creative ideas and untested approaches in isolated cases.
External Links
- Critical Appraisal Skills Programme (CASP) Checklist
- Critical Appraisal: A Checklist, 2016
- Dissecting the literature: the importance of critical appraisal, 2017
- Critical Appraisal of Clinical Research, 2017
- A guide to critical appraisal of evidence, 2019
- Critical appraisal: how to evaluate research for use in clinical practice, 2021
- University of Canberra Library - Evidence-Based Practice in Health
Comments
Post a Comment