Drug Metabolism
Introduction
Since lipophilic substances are not eliminated efficiently by the kidney, most lipophilic drugs are metabolized to more polar products before excreted in urine.
Liver is the principal site of drug metabolism, although other organs are involved (e.g. gut, kidneys, lung, skin and placenta).
Phase I and Phase II Reactions
Drug metabolism involves 2 kinds of reaction, which often occurs sequentially.
Phase I reactions (e.g. oxidation, reduction or hydrolysis) are catabolic, and the products are often more chemically reactive.
Phase II reactions are synthetic ("anabolic") and involve conjugation (i.e. attachment of a substituent group), which usually results in inactive products.
The P450 Monooxygenase System
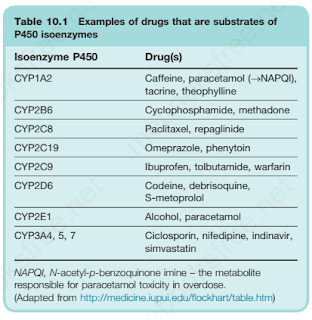
The Cytochrome P450 system comprises 57 isoenzymes, each derived from the expression of an individual gene.
- The nomenclature comprises a family number, a subfamily letter, and a number for an individual enzyme within the subfamily.
Each cytochrome 450 isoenzyme tends to metabolise a discrete range of substrates. Of the many isoenzymes, a few (CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4) appear to be responsible for the human metabolism of most commonly used drugs.
The genes that encode specific cytochrome 450 isoenzymes can vary between individuals and, sometimes, ethnic groups. These variations (polymorphisms) may affect metabolism of substrate drugs.
Drug Interactions
The effect of a cytochrome 450 isoenzyme on a particular substrate can be altered by interaction with other drugs. Drugs may be themselves substrates for a cytochrome 450 isoenzyme and/or may inhibit or induce the isoenzyme.
Presystemic ("First-Pass") Metabolism
Even if some drugs are well absorbed, they can be extracted so efficiently by the liver or gut wall that the amount reaching the systemic circulation is considerably less than the amount absorbed. This is known as presystemic (or first-pass) metabolism.
In other words, presystemic metabolism in liver or gut wall reduces the bioavailability of several drugs when they are administered by mouth.
Prodrugs
Prodrugs are taken by the patient in an inactive form and are converted by CYP450 enzymes into the active form. They are used by drug manufacturers to
- Extend the dosing interval
- Prevent drug abuse
Common prodrugs are
- Capecitabine → Fluorouracil
- Clopidogrel → Active metabolite
- Codeine → Morphine
- Colistimethate → Colistin
- Cortisone → Cortisol
- Famciclovir → Penciclovir
- Fosphenytoin → Phenytoin
- Levodopa → Dopamine
- Prednisone → Prednisolone
- Primidone → Phenobarbital
- Tramadol → Active metabolite
- Valacyclovir → Acyclovir
- Valganciclovir → Ganciclovir
NOTE: Nonetheless, safety concern can arise when a prodrug is used in patients with altered drug metabolism (e.g. due to genetics or a drug interaction).

Comments
Post a Comment