Haemophilia
Introduction
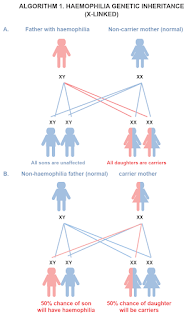
Haemophilia is a hereditary X-linked recessive bleeding disorder caused by a chromosomal mutation resulting a deficiency or absence of
- Coagulation factor VIII - Haemophilia A
- Coagulation factor IX - Haemophilia B
Female carrying a FVIII or FIX gene mutation are carriers. Some carriers of haemophilia experience bleeding problems, including joint haemorrhages, similar to males; in addition, they may experience problems that are specific to women, such as prolonged or heavy menstrual bleeding. Symptomatic carriers are considered to have mild or moderate haemophilia.
The incidence of
- Haemophilia A is about 1 in 5000 male births (more common)
- Haemophilia B is about 1 in 30,000 male births.
Clinical Diagnosis
Haemophilia should be suspected in individuals presenting with any of the following
- Spontaneous bleeding usually into joints, muscles and soft tissue
- Frequent bruising in early childhood
- Excessive bleeding following trauma or surgery
- Family history of bleeding
Screening tests for suspected hereditary bleeding disorders include
- Prothrombin time (PT)
- Activated Partial Thromboplastin Time (APTT)
- Platelet count
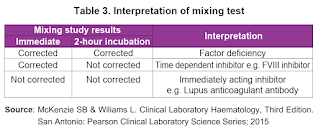
If APTT is prolonged and there is positive family history of haemophilia, proceed to perform FVIII or FIX factor assay. Otherwise, mixing study should be done first.
Clinical Presentation
The characteristic bleeding manifestations of haemophilia include
- Palpable ecchymosis
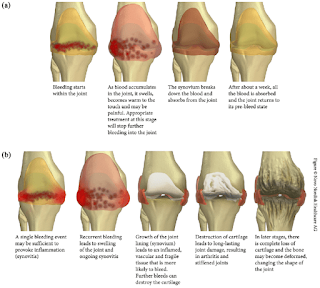
- Bleeding into joint spaces (hemarthroses)
- Muscle haemorrhages
- Excessive bleeding after surgery or trauma
The severity of clinical bleeding generally correlates with the degree of deficiency of either factor VIII or factor IX.
Sites of bleeding in haemophilia patient that are considered serious include
- Joints (hemarthrosis)
- Muscles, especially deep compartments (iliopsoas, calf, forearm)
- Mucous membranes of the mouth, nose and genitourinary tract
Bleeding is considered life-threatening and require immediate treatment if it occurs in the
- Neck or throat (including floor of the mouth)
- Intracranial
- Gastrointestinal
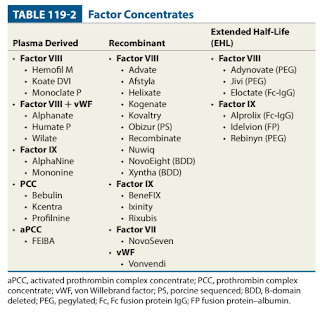
Factor Concentrate Replacement
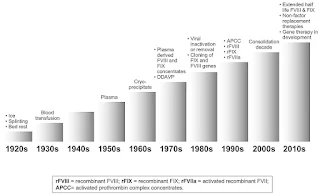
Therapy for haemophilia has undergone dramatic advances over the past few decades.
Intravenous factor replacement therapy for the treatment or prevention of bleeding is the mainstay of treatment for haemophilia.
- Since the mid-1980s, plasma-derived concentrates have been manufactured with a variety of virus-inactivating techniques including dry heat, pasteurization and treatment with chemicals (e.g. solvent detergent mixtures) to reduce the risk of transmission of hepatitis B, hepatitis C and human immunodeficiency virus (HIV).
- However, the current viral inactivation process does not eliminate a majority of the non-lipid viruses (e.g. hepatitis A virus, parvovirus, Creutzfeldt-Jakob). At the moment, the transmission is probably kept at a lower level by excluding at-risk blood donors.
- Recombinant factor VIII and IX is produced with recombinant DNA technology (NOT derived from blood donations), hence lower risk of infection transmission.
- Methods for prolonging the half-life of factor include pegylation and Fc fusion.
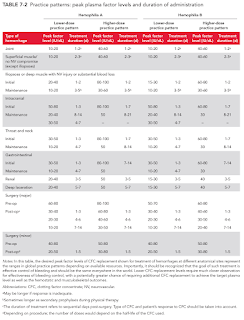
Factor replacement therapy should ideally be given as soon as the patient suspects a bleed and before the onset of overt swelling, loss of joint function and pain.
- Clotting factor concentrate should be administered immediately at a dose sufficient to raise the patient's factor level high enough to stop the bleeding.
NOTE: Malaysia CPG is based on the higher-dose practice pattern.
The following equation can be used to calculate an initial dose.
Factor Dosage (units) = (Desired level - Baseline level) x Weight (in kg) / k, where k is
- 2.0 for plasma-derived FVIII (haemophilia A) or 1.5 for recombinant FVIII
- 1.0 for plasma-derived FIX (haemophilia B) or 0.6 for recombinant FIX
The baseline level term can be omitted from the formula if it is negligible compared to the desired level.
NOTE: Doses of recombinant factor concentrate is higher than those of plasma-derived products to achieve equivalent plasma levels.
Prophylaxis Versus On-Demand Therapy
As recommended by the World Health Organization and the World Federation of Haemophilia, prophylactic factor replacement therapy for patients with severe haemophilia is considered the standard of care.
- The goal of this approach is to maintain a patient's minimum factor level at or above 0.01 units/ml (1%) with regular infusions of factor products.
- Prophylaxis prevented joint damage and decreased the frequency of joint and other haemorrhages.
- Challenges include high cost, inconvenience to families, possible difficulties with adherence, potential complications of central venous access and overtreat some patients.
There are many different prophylactic factor replacement therapy protocols but optimal regimen remains to be defined.
- Prophylactic regimens should be individualized according to bleeding phenotype, activity and pharmacokinetics.
On-demand therapy, also known as episodic therapy, is to administer the necessary factor only for acute bleeding episodes.
- Cessation of bleeding does not reverse the deleterious effects on synovial tissues by the blood which has accumulated in the affected joint.
Treatment of Inhibitors
Neutralizing antibodies to factors VIII and IX, known as inhibitors, develop in a subset of patients with haemophilia - the most serious complication of factor replacement therapy.
- Inhibitors are measured with the Bethesda assay, and titers are reported in Bethesda units (BUs).
- One BU is the amount of inhibitor needed to inactivate half of the factor VIII or factor IX in a mixture of inhibitor-containing plasma and pooled normal plasma.
The lifetime risk of inhibitor development is
- 20-30% in severe haemophilia A
- 5-10% in mild or moderate haemophilia A
- <5% in haemophilia B
Risk factors for inhibitor development are
- High intensity treatment of clotting factor within the first 8-12 weeks of therapy.
- Genotype with large deletions and nonsense mutations
- Family history of inhibitors
- In a retrospective cohort study, regular prophylactic therapy reduced the risk of inhibitor development by 60% compared with on-demand therapy. However, the Cochrane systematic review showed no significant difference.
- Plasma-derived factor containing von Willebrand factor had a lower incidence of inhibitor development than those treated with recombinant factor VIII - no clear and consistent evidence yet.
The presence of an inhibitor is suspected when a decreased clinical response to factor replacement is observed or it may be discovered incidentally on routine laboratory screening. Inhibitor should be screened
- At regular intervals
- Children - once every 5 exposure days until 20 exposure days, then every 10 exposure days between 21 and 50 exposure days, then at least twice a year until 150 exposure days.
- Adults with >150 exposure days - every 6-12 months
- After intensive treatment for >5 days, within 4 weeks of the last infusion
- Prior to surgery
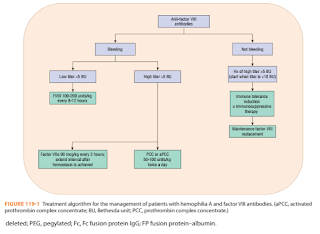
Patients with inhibitors to factor VIII or factor IX are divided into 2 groups.
- Low levels of inhibitors (<5 BU/ml) - have little or no rise in antibody titers after exposure to the factor.
- High responders (>5 BU/ml) - have higher inhibitor levels and develop an increase in antibody titer after exposure (anamnestic response).
Therapy for patients with inhibitors involves
- Treatment of acute bleeding episodes
- For patients with a low inhibitor titer, the administration of high doses of the specific factor often can control bleeding episodes. 2-3 times the usual replacement dose and more frequent dosing intervals are often necessary to overcome the antibody.
- In patients with high-titer inhibitors, consider bypassing agents such as PCCs, activated prothrombin complex concentrates (aPCCs) (Brand: Feiba) and recombinant factor VIIa (Brand: NovoSeven).
- rFIIa and aPCC are equally effective and well tolerated with no increase in thromboembolic risk in the treatment of acute bleeding episodes in haemophilia with inhibitors.
- In a patient with a newly diagnosed inhibitor, it is prudent to use recombinant factor VIIa because aPCCs contain a small amount of factor VIII or IX and have been shown to increase the inhibitor titer.
- A drawback with recombinant factor VIIa is the product's short half-life, which necessitates initial dosing every 2 hours.
- Because no laboratory test measures the effectiveness of bypassing therapy in patients with inhibitors, close monitoring for worsening or resolution of symptoms is essential.
- Treatment directed at eradicating the inhibitor
- Immune tolerance induction (ITI) involves the regular infusion of factor VIII to induce antigen-specific tolerance. Nonetheless, ITI protocols vary widely (e.g. Bonn protocol, Dutch protocol and Malmo protocol).
- Initiation of ITI should be postponed until the inhibitor titre has dropped to <10 BU to improve the likelihood of success. Other prerequisites for starting ITI to ensure no interruption of treatment for best response include
- Commitment from haemophilia patient with inhibitor and caregiver
- Good venous access
- Adequate budget
- This approach is not recommended for patients with haemophilia B who have developed inhibitors due to the risk of hypersensitivity reactions and anaphylaxis associated with factor IX administration in this group.
- Serious thrombotic complications including pulmonary emboli, deep-vein thrombosis and myocardial infarction have been associated with the use of PCCs and aPCCs.
- Although not commonly used in ITI protocols, immune modulation can improve tolerance success. Agents such as cyclophosphamide, intravenous immune globulin, rituximab.
Non-factor Replacement Therapies
Emicizumab is a recombinant, humanized, bispecific monoclonal antibody that bridges activated factor IX and factor X, performing the function of the missing activated factor VIII in maintaining haemostasis.
- Approved for prophylaxis therapy in patients with haemophilia A (with or without inhibitors) (NOT intended for treatment of acute bleeding episodes)
- Administered in a single subcutaneous injection once weekly.
Concizumab (inhibiting tissue factor pathway inhibitor) and fitusiran (lower anti-thrombin level) are being investigated as alternative prophylaxis for haemophilia patients with inhibitors.
Gene Therapy
Haemophilia is an excellent candidate for gene therapy because tight control of gene expression is not required. Even low levels of factor expression can reduce bleeding episodes in patients with severe haemophilia.
The goal is to achieve a sustainable factor activity level of over 5%, which is sufficient to convert patients with severe disease to a much milder phenotype.
- If a treatment strategy could produce consistent factor activity levels of around 50%, it would be considered curative.
Possible drawbacks to gene therapy include a risk of inhibitor formation, tumorigenesis related to possible integration of the viral vector, possible germline transmission of the viral vector, and concerns about long-term gene expression.
Other Pharmacologic Therapy
Treatment with desmopressin acetate often is adequate for minor bleeding episodes in patients with mild haemophilia A or symptomatic carrier of haemophilia A.
- A single dose of DDAVP either via IV or SC route can be expected to boost the level of FVIII to 3-6 folds higher than the baseline. There is however some variability in response and a test dose is the only way to distinguish good responders from poor or non-responders.
- Should not be used as primary therapy for life-threatening bleeding episodes such as intracranial haemorhage or for major surgical procedures.
- Relatively contraindicated in children <2 years old who may be at risk of seizure secondary to cerebral oedema due to hyponatremia.
Antifibrinolytic agents (e.g. tranexamic acid) are particularly beneficial for the treatment of oral bleeding. It can also be helpful adjuvant therapy in GI bleeding, epistaxis and menorrhagia.
- It should be used with caution in patients with urinary bleeding, due to the risk of obstruction and subsequent renal toxicity.
- Concomitant use of prothrombin complex concentrates (PCCs) and antifibrinolytics should be avoided because of the risk for thrombosis.
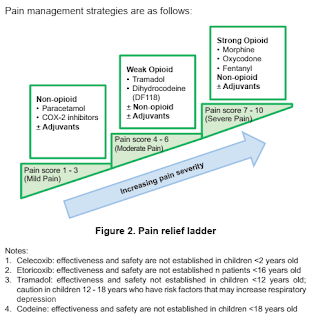
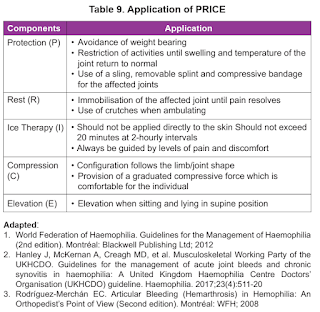
Pain, both acute and chronic, can be a common occurrence in patients with haemophilia. The most likely cause of acute pain is bleeding, and treatment should include factor replacement to stop the bleeding and PRICE (Protect, Rest, Ice, Compression and Elevation).
Non-pharmacological Treatment
Rehabilitation of musculoskeletal system.
Applying PRICE principles.
Non-contact sports (e.g. walking, swimming and cycling) or physical activity should be encouraged.
Weight management.
Good oral hygiene to prevent dental caries.
Avoid antiplatelet agents (particularly aspirin and non-selective NSAIDs unless strong indication) and intramucular injections.



Comments
Post a Comment