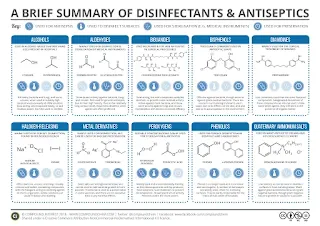
Antiseptics
Introduction
An antiseptic is a disinfectant that is used on skin and other living tissues, thereby limiting or preventing infection.
- Disinfectants (which is used on non-living surfaces) tend to have higher concentrations, which are not suitable for use on the skin or mucous membranes.
- Different from topical antibacterials, antiseptics can kill or prevent the growth of viruses, bacteria and fungi. Plus, antiseptics are less likely to develop resistance to microbes, a concentrated localized effect, are generally better tolerated, and are widely available.
Commonly used antiseptics include
- Cetrimide
- Chlorhexidine
- Hydrogen peroxide
- Povidone-iodine
- Sodium hypochlorite
Roles in Wound Care
Antiseptics are suitable for cleaning contaminated or traumatic acute wounds and bites.
The routine use of antiseptics on previously cleansed and dressed wounds is controversial.
- Although antiseptics reduce microbial concentrations, wounds do not necessarily have to be sterile to heal.
- Antiseptics can interfere with wound repair if they are toxic to the cells involved in the wound healing process (e.g. keratinocytes and fibroblasts). Some antiseptics are inactivated by organic material such as pus and necrotic tissue, which limits their efficacy.
NOTE: Irrigation with normal saline or tap water will clean most wounds adequately.
Acriflavine
The acridine derivatives are slow-acting antiseptics.
- They are bacteriostatic against many Gram-positive bacteria but less effective against Gram-negative bacteria.
- They are ineffective against spores.
- Prolonged treatment may delay healing.
Cetrimide
Cetrimide is a quaternary ammonium antiseptic with actions and uses typical of cationic surfactants.
- It is rapidly inactivated by organic matter, can cause sensitivity, and should generally only be used when other options are unavailable
Hydrogen Peroxide
Hydrogen peroxide is an oxidising agent used as an antiseptic, disinfectant, and deodorant. It has weak antibacterial activity and is also effective against viruses, including HIV. It also has a mild haemostatic action.
- It owes its antiseptic action to its ready release of oxygen when applied to tissues, but the effect lasts only as long as the oxygen is being released and is of short duration; in addition, the antimicrobial effect of the liberated oxygen is reduced in the presence of organic matter.
- The mechanical effect of effervescence is probably more useful for wound cleansing than the antimicrobial action.
- It can dissolve clots and cause bleeding, and is no longer widely used.
Povidone Iodine
Povidone-iodine is an iodophore that is used as a disinfectant and antiseptic mainly for the treatment of contaminated wounds and pre-operative preparation of the skin and mucous membranes as well as for the disinfection of equipment.
- Iodophores are loose complexes of iodine and carrier polymers. Solutions of povidone-iodine gradually release iodine to exert an effect against bacteria, fungi, viruses, protozoa, cysts, and spores; povidone-iodine is thus less potent than preparations containing free iodine but it is less toxic.
Aqueous antiseptic solutions contain 5-10% povidone-iodine. For irrigating open wounds, povidone-iodine can be left on the skin for 3-4 minutes and then washed off.
- The application of povidone-iodine to severe burns or to large areas otherwise denuded of skin may produce the systemic adverse effects associated with iodine and metabolic acidosis, hypernatraemia, and renal impairment.
- Regular or prolonged use should be avoided in patients with thyroid disorders or those receiving lithium therapy.
Sodium Hypochlorite
Sodium hypochlorite is a disinfectant and antiseptic with the brief and rapid actions of chlorine.
Hypochlorite solutions are now generally considered to be too irritant for use in the management of wounds
- Sodium hypochlorite is available in commercial products such as Eusol, Milton and Dakin’s Solution.
- Studies suggest that they may delay wound healing if repeatedly applied to open wounds.
Comments
Post a Comment