Drug Discovery and Development
Introduction
Drug development is an expensive and lengthy process, taking about 2-3 billion dollars and over 10 years to discover and develop a drug to commercialization.
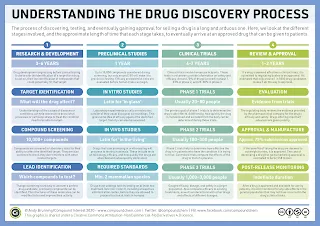
Drug Development Timeline
Discovery and Development
- Research for a new drug begins in the laboratory.
- Typically, new drugs are discovered through new insights into a disease process, unanticipated effects of existing treatment and tests of many novel molecular compounds.
- Lead optimization studies are carried out to identify compounds with the "best" properties (activity, toxicity, pharmacokinetic, physiochemical, etc.)
Preclinical Research
- Drugs undergo laboratory (in vitro) and animal (in vivo) testing to answer basic questions about safety.
Clinical Research
- Since animal models are not a substitute for studies of how the drug will interact with the human body, drugs are then tested on humans to make sure they are safe and effective.
- Clinical trials
- Phase I - Initial human safety assessment
- Phase II and III - Determine efficacy and further safety
Drug Registration
- FDA review teams thoroughly examine all of the submitted pre-clinical and clinical data related to the drug and make a decision to approve or not approve it.
Post-Market Safety Monitoring
- Post-marketing surveillance to ensure continued safety and efficacy upon widespread use in the public.
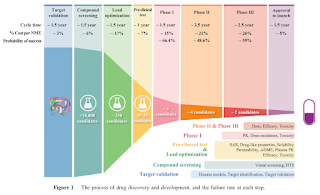
High Failure Rate of Drug Development
Analyses of clinical trial data from 2010 to 2017 show that 90% of clinical drug development failures are attributable to 4 possible reasons- Lack of clinical efficacy (40-50%)
- Unmanageable toxicity (30%)
- Poor drug-like properties (10-15%)
- Lack of commercial needs and poor strategic planning (10%)
Summary
The drug development process is a complex and challenging one.
- However, it is also a rewarding one, as new drugs can save lives and improve the quality of life for millions of people.
Comments
Post a Comment