Metered-Dose Inhalers (MDIs)
Introduction
Metered-dose inhalers (MDIs) were introduced in the mid-1950s and are the most commonly used inhalation drug delivery devices.
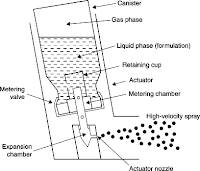
- In MDIs, drug is either dissolved or suspended in liquid propellant(s) together with other excipients, including surfactants, and presented in a pressurized canister fitted with a metering valve.
Shaking The Canister
Pressurized aerosols may be formulated as either solutions or suspensions of drug in the liquefied propellant.
- In practice, pressurized inhaler formulations have traditionally been almost exclusively suspensions. Hence, all the problems of conventional suspension formulation, such as caking, agglomeration, particle growth, etc. need to be considered.
Consequently, suspension-based MDIs should be shaken prior to use, but not the solution-based.
- Not shaking an MDI canister that has been standing overnight decreases total and respirable dose by 25% and 35% respectively, because the drugs in MDI formulations are usually separated from the propellants when standing.
Priming of Inhaler Devices
Priming should be done for new inhaler devices and when the inhaler has not been used for a while.
- To illustrate, when a metered-dose inhaler (MDI) is new or has not been used for a while, the drug may be separated from the propellant and other ingredients in the canister and metering valve.
- Because shaking the metered-dose inhaler will mix the suspension in the canister but not the metering chamber, priming of the MDI is deemed to be required.
Handling of Inhaler Devices, 2010 suggested that re-priming should be done for MDIs that are not used for more than 2 weeks.
- However, based on the product leaflet, the recommendations on re-priming of each metered-dose inhalers on the market are more complicated. This is well illustrated in A Guide to Aerosol Delivery Devices for the Respiratory Therapist.
Local product leaflet suggests the following.
- Alvesco - 3 puffs if the inhaler has not be used for 1 week or more.
- Flixotide - 2 puffs if the inhaler has not been used for a week or more.
- Flutiform - 4 puffs if the inhaler has not been used for 3 days or more.
- Foster - 1 actuation if the inhaler has not been used for 14 days or more.
- Seretide - 2 puffs if the inhaler has not been used for a week or more.
- Symbicort Rapihaler - 2 actuations if the inhaler has not been used for more than a week.
- Ventolin - 2 puffs if the inhaler has not been used for 5 days or more
MDI Inspiratory Force
Among the inhalers, metered-dose inhaler (MDI) is one of the hardest to be mastered.
- It requires good hand coordination with inhalation. Some patients actuating the inhaler too late while inhaling and consequently, you can see visible smokes coming out from the mouth.
- From experience, I found it is sometimes more helpful to ask the patients to inhale slowly first and then actuate the inhalers, especially in elderly. The concept of simultaneous inhalation and actuating is not as simple as we first thought.
Even so, this brings us to another question: is inhaling with no smoke escaping from the mouth afterward equivalent to satisfactory MDI technique?
- Well, it is not a clear "Yes".
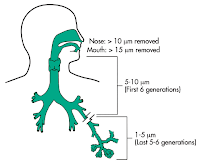
Ideally, the drug particles are expected to reach the lung periphery to give an optimal effect. For simplicity, there is a total of 3 main mechanisms of aerosol deposition:
- Inertial impaction
- Gravitational sedimentation
- Diffusion
Apart from particle size, particle velocity and settling time play an important in lung deposition.
- If we are breathing in strong and at a fast rate, much of the drugs will be trapped at the oropharyngeal region. Hence, it is particularly important for us to highlight that a slow yet deep inhalation is needed in using MDI.
- To allow deep inhalation, exhale slowly and completely through mouth away from inhaler first.
- Undoubtedly, it is equally important to hold the breath for 6 to 10 seconds or as long as possible to allow for gravitational sedimentation of the drug particles.
In December 2018, U.S. Food and Drug Administration has approved ProAir Digihaler, the first and only digital inhaler with built in sensors.
- These sensors can detect when the inhaler is used and more importantly, can measure inspiratory flow rate.
- The second function is the one that caught my interest.
Dose Checking of MDI
Frankly speaking, it is hard to manually determine the remaining contents of a metered-dose inhaler.
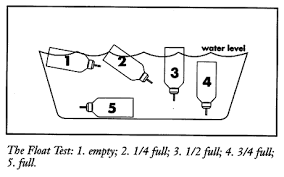
It used to be suggest floating the canister in water. However, this floating/immersion technique is no longer endorsed since it can reduce the ability of the MDI to work properly.
Moreover, even after the MDI delivers the number of puffs stated on their label, it may look, taste and feel like it is still working, but the dose delivered may be very low. This "tailing off effect" may last long after the MDI is empty of drug.
By keeping a spare one, the shaking method can be done to estimate the remaining contents of the MDI canister but it still does not reflect the actual content of the canister.
The only reliable method to determine the number of doses remaining in an MDI is counting the doses given either manually or with a dose counter.
- A manual counting may be impractical and undependable, especially in patients who use reliever medications on the go.
- Hence, US FDA requires new MDI to have integrated dose counters and recommends that all MDIs to have dose counting devices that indicate when the MDI is approaching its last dose.
- In August 2020, it is announced that in Australia, Ventolin inhalers with a dose counter are now listed on the PBS, with a full transition to the new design expected by end of 2020.
Cleaning of MDIs
It is important to keep the device clean to prevent medication accumulation and prevent blockage over the nozzle.
- Remove the canister.
- Clean the plastic parts of the inhaler by rinsing it under running tap water for about 30 seconds.
- Do not wash or put the canister in the water.
- Air-dry the plastic parts (Do not wipe).
- Reassemble the inhaler.
However, based on product inserts, certain MDI canisters should not be removed from the actuator and the mouthpiece should not be cleaned with water (just wipe the outside and inside of the mouthpiece with a dry cloth), such as Foster, Seretide, Symbicort Rapihaler and Trimbow.
Spacer
A spacer looks like a holding chamber and is recommended for small children and elderly who is having difficulty with using MDI.
- All the latest research shows that a puffer with spacer works just as well as a nebulizer for treating asthma symptoms.
- A 2017 study showed the use of soft mist inhaler (i.e. Respimat) with a valved holding chamber should deliver a similar fine particle mass of active pharmaceutical ingredient compared to soft mist inhaler alone.
Aerochamber Plus Flow-Vu
OptiChamber Diamond
NOTE: Both the AeroChamber Plus Flow-Vu (adult sizes) and OptiChamber Diamond feature a high-flow alert whistle that sounds when inhalation is too rapid, prompting slower breathing. Ideally, these chambers should also be replaced every 12 months for optimum medication delivery.
Have you ever wonder how should we use a plastic spacers with no antistatic property?
- Different from spacers made from antistatic polymers (which can be used right after opening the package), the new plastic spacers need to be washed before you use them for the first time.
- Electrostatic surface charge on new standard plastic spacers can be reduced by washing the plastic spacer in dishwashing liquid and allowing it to air dry or drip-dry without wiping (i.e. suds intact).
- If a new spacer has to be used immediately, you can "prime" the spacer by firing multiple puffs (at least 10, however the optimal number of actuations for priming is not known) into it to begin with to help reduce the static build-up inside. You can then take your medication dose as usual.
NOTE: When cleaning the chamber, remove excess water by shaking and allow it to air-dry in a vertical position. Wiping the chamber dry can lead to electrostatic buildup.
Soft-Mist MDI
The Respimat is a propellant-free soft-mist inhaler.
- The Respimat utilizes mechanical energy in the form of a tensioned spring to generate the soft aerosol plume.
- Since the device is propellant free, there is no need to shake it.
Similar to conventional MDIs, the Respimat will need to be primed before use at times and at times when the device has had no use.
- If not used for more than 7 days, actuate the inhaler once towards the ground.
- After more than 21 days of no use, it is recommended to actuate the device until aerosol is seen, then actuate 3 times more.
Maintenance.
- Clean up the Respimat mouthpiece with damp cloth or tissue daily after use.
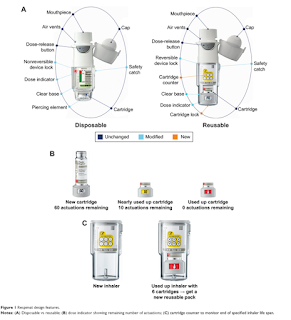
- The discard by date is 3 months from the date the cartridge is inserted into the inhaler.
- For Respimat re-usable, replace the inhaler once you have used an inhaler with 6 cartridges.
- The functioning of the Respimat re-usable inhaler has been demonstrated in tests for 540 actuations (corresponding to 9 cartridges).
External Links
- NPS Inhaler Technique, 2020
- National Asthma Council Australia - Puffer and inhaler care
- Australian Asthma Handbook - Use and care of spacers
- National Asthma Council Australia - Spacer use and care
- Reducing electrostatic charge on spacer devices and bronchodilator response
- Delivery of Respimat Soft Mist inhaler (SMI) from a Valved Holding Chamber (VHC), 2017
- A Guide to Aerosol Delivery Devices for the Respiratory Therapist, 2018
.png)

Comments
Post a Comment