Entresto
Introduction
Graduating from university is not the ending of learning, but the beginning of self-learning.
Never in my university years, I have heard of the term ARNI (angiotensin-receptor nerilysin inhibitors). An example of it is Entresto, which consists of sacubitril and valsartan in a fixed combination.
- Valsartan, in formulation, is more bioavailable than other tablet formulations; 26 mg, 51 mg and 103 mg valsartan is equivalent to 40 mg, 80 mg and 160 mg respectively.
Entresto is a novel therapy recommended for patients with
- Chronic heart failure with reduced ejection fraction (HFrEF) to reduce morbidity and mortality
- Essential hypertension
What Benefits does Entresto Offer over ACEI in Heart Failure?
A randomized double-blind trial (PARADIGM-HF) in patients with HFrEF found that sacubitril-valsartan (Entresto) reduced all-cause mortality as well as cardiovascular mortality and hospitalization for HF compared with a proven dose of the ACE inhibitor enalapril.
How about Safety Data?
The safety and efficacy of sacubitril-valsartan initiation during hospitalization for acute HF was evaluated in the PIONEER-HF trial, in which 881 patients hospitalized with acute HF were randomly assigned to receive either sacubitril-valsartan or enalapril following hemodynamic stabilization and followed for eight weeks.
- The initiation of sacubitril-valsartan therapy led to a greater reduction in the NT-proBNP concentration than enalapril therapy with similar risk of worsening renal function, hyperkalaemia, symptomatic hypotension and angioedema.
- Of note, criteria for hemodynamic stability in PIONEER-HF included systolic blood pressure ≥100 mmHg, no intravenous vasodilators or increase in intravenous diuretics in the preceding six hours, and no inotropes in preceding 24 hours.
Treatment of Hypertension
The phase III pivotal trial for Japanese patients with mild or moderate essential hypertension assessed Entresto in comparison to the angiotensin II receptor blocker (ARB) olmesartan.
- The results showed that treatment with Entresto at 200 mg OD is more effective than olmesartan 20 mg OD and generally well tolerated.
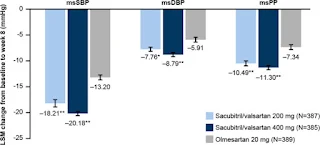
The superiority of systolic blood pressure lowering effect of both Entresto 200 mg once daily and 400 mg once daily compared to olmesartan 20 mg once daily was also seen in an earlier randomized, double-blind, 8-week study in Asian patients.
NPRA Safety Warning: Risk of Psychiatric Events
It has been observed that the use of sacubitril/valsartan has been linked to psychiatric events such as hallucination, paranoia and sleep disorders.
- The time to onset was reported to be between 2-7 days after starting treatment or after dose escalation.
- The exact mechanism on how sacubitril/valsartan causes these psychiatric events are not known. However, it was postulated that neprilysin blockade may contribute to the inhibition of beta-amyloid degradation, a protein that is involved in the pathogenesis of neurodegenerative diseases.
Contraindications
Contraindications to the use include
- Pregnancy (given risk of foetal toxicity including foetal death)
- History of angioedema (of any cause)
- Severe hepatic impairment, biliary cirrhosis and cholestasis
External Links
- 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure
- Sacubitril/valsartan: evaluation of safety and efficacy as an antihypertensive treatment, 2018
- Efficacy and safety of sacubitril/valsartan compared with olmesartan in Asian patients with essential hypertension: A randomized, double-blind, 8-week study, 2019
- Sacubitril/valsartan: Risk of psychiatric events, 2021
- Efficacy of sacubitril/valsartan versus olmesartan in Japanese patients with essential hypertension: a randomized, double-blind, multicenter study, 2022
Wheelchairs and mobility solutions are essential tools for individuals with mobility impairments, enabling them to maintain independence and improve their quality of life.
ReplyDeleteFolding Wheelchair
Lightweight Wheelchair
Manual Wheelchair
Wheelchair In India