Safe Handling of Hazardous Drugs
Introduction
As stated in the Procedures for Developing the NIOSH List of Hazardous Drugs in Healthcare Settings (Procedures), NIOSH defines a hazardous drug as a drug that is
- Approved for use in humans by FDA CDER,
- Not otherwise regulated by the U.S. Nuclear Regulatory Commission, and
- Either
- Is accompanied by prescribing information in the "package insert" that includes manufacturer's special handling information (MSHI) to protect workers handling the drug, or
- Is identified as a carcinogenic hazard, development hazard, reproductive hazard, genotoxic hazard, or other health hazard by exhibiting one or more of the following toxicity criteria in humans, animal models or in vitro systems.
- Carcinogenicity
- Developmental toxicity (including teratogenicity)
- Reproductive toxicity
- Genotoxicity
- Organ toxicity at low doses, or a
- Structure and toxicity profile that mimics existing drugs determined hazardous by exhibiting any one of the previous five toxicity types.
However, if a drug also exhibits a molecular property that may limit the potential for adverse health effects from exposure to the drug in healthcare workers, it may be determined it is not a hazard.
Developing A Facility-specific List of Hazardous Drugs
The NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings, 2016 included 3 groups of drugs:
- Group 1: Antineoplastic drugs
- Group 2: Non-antineoplastic drugs that meet one or more of the NIOSH criteria for a hazardous drug (including reproductive risk)
- Group 3: Drugs that primarily pose a reproductive risk to men and women who are actively trying to conceive and women who are pregnant or breastfeeding, because some of these drugs may be present in breast milk.
However, the NIOSH List of Hazardous Drugs in Healthcare Settings, 2024 no longer uses "antineoplastic" as a table descriptor in the title of Table 1 and does not have a separate table (previously Table 3) for drugs that may pose only a developmental and/or reproductive hazard (currently also listed in Table 2).
- Table 1 now includes drugs that have MSHI in the package insert and/or meet the NIOSH definition of a hazardous drug and one or more of these criteria:
- Are classified by the National Toxicology Program (NTP) as "known to be a human carcinogen"
- Are classified by the International Agency for Research on Cancer (IARC) as Group 1 "carcinogenic to humans" or Group 2A "probably carcinogenic to humans"
- Table 2 now contains drugs that meet one or more of the criteria in the NIOSH definition of a hazardous drug and
- Do not have MSHI
- Are not classified by the NTP as "known to be a human carcinogen"
- Are not classified by the IARC as Group 1 "carcinogenic to humans" or Group 2A "probably carcinogenic to humans"
NOTE: Some drugs in Table 2 may also have adverse developmental (including teratogenicity) and/or reproductive effects.
The list is an aid designed to enable employers to identify which drugs handled by employees are considered by NIOSH to be hazardous drugs.
- Because new drugs and new formulations are continuously brought to market between NIOSH's periodic updates, hazardous drug evaluation should be a continual process.
- Employers should establish their own procedures to identify and evaluate new drugs as they enter their workplace and, when appropriate, reassess their presence on hazardous drug lists as toxicological data become available to support re-categorization
Health Hazards and Possible Health Risks
The chance that hazardous drugs will harm healthcare workers and the severity of the harm depend on the
- A drug's toxicity refers to the harm it can cause to a person's health.
- Drug formulation refers to the form the drug takes - such as capsules, tablets, powders, liquids, creams, transdermal patches or prefilled syringes.
- Route of exposure applies to how workers may be exposed to the drug, such as through inhalation, absorption through skin or mucous membranes, ingestion or accidental injection.
- Workplace activity involves how healthcare workers use and handle the drug in the workplace - such as opening shipments, compounding, administering or cleaning up after use or spills.
Exposure to hazardous drugs has been associated with many adverse health effects, including
- Ocular irritation, headache, cough, dizziness, nausea and vomiting, skin rashes
- An increase in the risk of leukaemia and other cancers,
- A risk of damage to organs or organ systems, and
- A risk to the ability to reproduce (successfully conceive and have healthy babies).
The Hidden Truth
Studies have shown that the antineoplastic residues can be found be on the external packaging of both oral and IV drugs.
However, till today, most of us still have the misconception that the personnel who exposed to hazardous drugs are mainly the pharmacists who are reconstituting intravenous cytotoxic drugs or nurses who administer the hazardous drugs to patients.
- Studies have proven the otherwise. Even unit clerks, transport workers, ward aides, dietitians and oncologists were observed touching contaminated surface and traces of hazardous drugs can actually be found in their urine.
Key Issues in Handling Hazardous Drugs
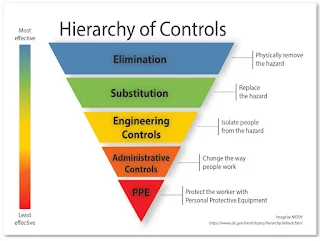
Engineering controls
- Use of biological safety cabinet (BSCs) and compounding aseptic containment isolators (CACIs), closed system drug-transfer devices (CSTDs), robotic systems and needleless systems for compounding sterile and nonsterile hazardous drugs.
- Establish negative pressure relative to surrounding areas to contain hazardous drugs and minimize the potential of exposure.
Administrative controls
- Personnel training.
- Medical screening and surveillance and alternative duty.
- Limit the time workers handle hazardous drugs.
- Mandate handwashing with soap and water before eating, drinking, smoking, using the bathroom, removing or applying cosmetics, or leaving the workplace.
- Prohibit consumption of food and drink, chewing gum, using tobacco or applying cosmetics in the areas where workers handle hazardous drugs.
- Immediate accessibility of spill kits in areas where workers use hazardous drugs. Consider having spill-response exercises or drills.
- Segregation of hazardous drugs inventory from other drug inventory.
- Oral hazardous drugs
- No crushing or compounding may be done in an unprotected environment.
- Prohibit the use of automated counting machines for hazardous drugs.
- Wearing chemotherapy gloves (ASTM D6978-tested gloves)
- Use powder-free gloves because the powder can contaminate the work area and might adsorb and retain hazardous drugs.
- Double gloving requires 1 glove to be worn under the cuff of the gown and the second glove over the cuff.
- Change gloves every 30 minutes during compounding or immediately when damaged or contaminated, unless otherwise recommended by the manufacturer's documentation.
- When removing gloves, turn them inside out so that contaminated surfaces do not touch uncontaminated surfaces.
- Washing hands thoroughly with soap and water after removing and disposing of gloves.
- Gowns must be designated for single use, be disposable and be shown to resist permeation by the types of hazardous drugs used.
- Disposable gowns made of polyethylene-coated polypropylene or other laminate materials offer better protection than those made of noncoated materials.
- If no permeation information is available for the gowns used, change them every 2-3 hours or immediately after a spill or splash.
- Use a fit-tested N95 respirator for routine handling of hazardous drug if there is potential for inhalation of fine powders.
- A surgical mask does not offer respiratory protection.
- The mat should be changed immediately if a spill occurs and regularly during use and should be discarded at the end of the daily compounding activity.
- Head, hair, shoes and sleeve covers
Summary
The following are a few key references that explore the safe handling of hazardous drugs:
- USP General Chapter <800> Hazardous Drugs-Handling in Healthcare Settings, 2023
- ASHP Guidelines on Handling Hazardous Drugs, 2018
- NIOSH List of Hazardous Drugs in Healthcare Settings, 2024
- ISOPP Standards for the Safe Handling of Cytotoxics, 2022
External Links
- Packaging and Surface Contamination with Antineoplastic Drugs in a Hospital Pharmacy in Sweden, 2005
- Hazardous drug residue on exterior vial surfaces: evaluation of a commercial manufacturing process, 2014
- USP General Chapter <800> Hazardous Drugs-Handling in Healthcare Settings, 2023
- ASHP Guidelines on Handling Hazardous Drugs, 2018
- NIOSH List of Antineoplastic and Other Hazardous Drugs in Healthcare Settings, 2016
- NIOSH List of Hazardous Drugs in Healthcare Settings, 2024
- Hazardous drugs: NIOSH update impact on pharmacy, 2020
- ISOPP Standards for the Safe Handling of Cytotoxics, 2022
- Managing Hazardous Drug Exposures: Information for Healthcare Settings, 2023
- Procedures for Developing the NIOSH List of Hazardous Drugs in Healthcare Settings, 2023
Comments
Post a Comment