Functional Groups
Introduction
Functional group is an atom or group of atoms that is part of a larger molecule (linked through covalent bond) and that has a characteristic reactivity, i.e.
- Affecting physical properties of a compound
- As a sites of chemical reaction.
It also forms the basis for naming organic compounds.
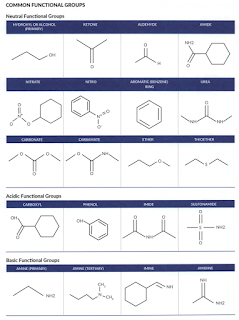
Common Functional Groups
Some of the common functional groups are depicted in the table below.
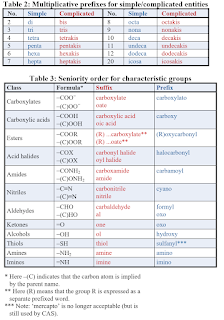
IUPAC Nomenclature
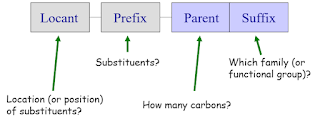
Naming of organic compounds have the following 4 main part.
Approach
- Identify the longest carbon chain containing the most important functional group as the parent.
- Name any substituents attached and arrange them in alphabetical order.
- Specify substituent location by numbering the parent chain from the end closest to the functional group.
- Use hyphens to separate numbers and letters.
- Use commas to separate numbers from numbers.
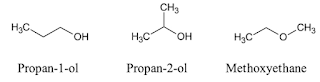
Isomers
Isomers are compounds possessing identical molecular but different atomic arrangements. This arrangement can result in different functional groups.
Structural isomers (or constitutional isomers) possess the same molecular formula but different bond connectivity.
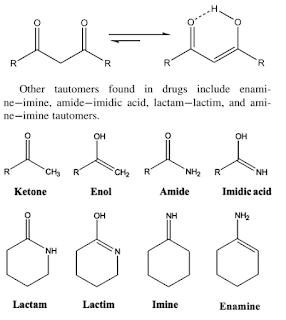
- Tautomers are a unique type of structural isomer that occurs within a single molecule resulting from resonance through a π bond system. The π bond system acts as an electron conductor, allowing electrons to flow in different directions depending on the environment and needs of the system.
Stereoisomers are isomers that contain the same molecular formula with identical atom connectivity.
- Optical isomers
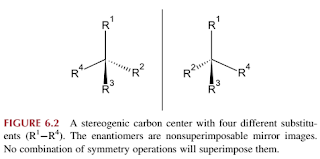
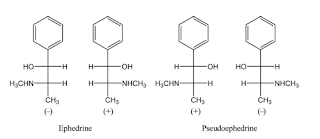
- Enantiomers are chiral stereoisomers that are mirror images of each other, lack an internal plane of symmetry, and therefore are nonsuperimposable. They have identical physical and chemical properties (such as melting and boiling point).
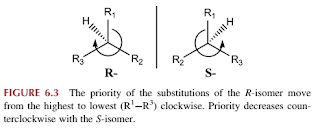
- When the priority of the three remaining substituents decreases in a clockwise rotation, it is labelled R-, for rectus, Latin for right. If the decrease priority is counterclockwise, it is labelled S-, for sinister, Latin for left.
- The enantiomer rotating polarized light clockwise is designated (+) or dextrorotary (d-), while that rotating counterclockwise is (-) or levorotary (l-). An equal (1:1) mixture of two enantiomers, known as racemic mixture, has zero net rotation.
- There is no fixed relationship between R/S and D/L labelling.
- Diastereomers are stereoisomers that are not mirror image of one another. It is commonly associated with chiral isomers, possessing 2 or more stereogenic centers. They typically have distinct physical properties from each other.
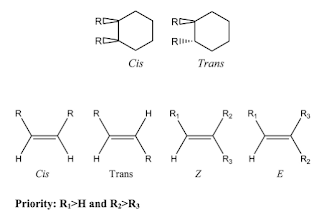
- Geometric isomers
- Geometric isomerism occurs as a result of restricted rotation around a bond, commonly resulting from a double bond or ring system. Geometric isomers typically have distinct physiochemical and biological properties.
- Cis is the designation used when the identified groups are on the same side of the plan of symmetry of the bond of interest. In trans, these groups are on opposite sides of this plane.
- Under normal conditions, these forms are stable and cannot interconvert. However, when under certain high-energy conditions, cis/trans-isomerization can occur.
- Trans isomers are typically more stable than cis due to less steric interactions between substituents.
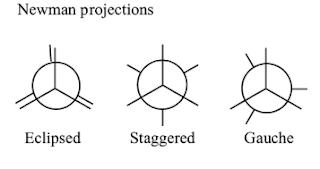
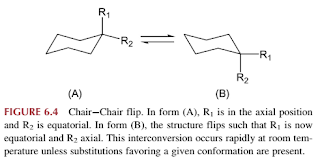
- Conformational isomers
- Conformational isomerism is a type of stereoisomerism resulting from nonidentical spatial arrangement of atoms attached to another atom via single bonds.
Summary
While the intricacies of drug design may not be the primary focus for most pharmacists, recognizing functional groups provides a crucial foundation for understanding drug binding, targeting, and overall mechanisms of action.
- Drugs are designed to interact with target receptors through specific functional groups, acting like keys that fit precisely into locks.
Comments
Post a Comment