Drug Nomenclature
Introduction
Drug nomenclature has evolved over time.
- In the past, drug names were formed by condensing their chemical names.
Types of Drug Nomenclature
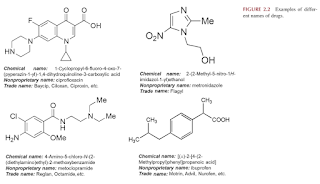
Most drug entities are known by several chemical names, code number, several trivial designations, a formally selected nonproprietary name, and one or more proprietary (trademark) names.
- Code name - The first name given to a drug by the manufacturer during the early product development. It is usually a letter abbreviation, a number or a combination of both.
- Chemical names - Scientific names of the drug based on the arrangement of atoms in their molecules and their functional groups (molecular structure).
- Generic or nonproprietary names - Names given to drug molecules after they have shown significant efficacy, tolerability and commercialization potentials. The name is usually simple, concise and meaningful.
- Proprietary, trade or brand names - Names given by the innovator company of the drug. The first letter of the trade name is written in capital and distinguished by superscript "®" or "™" at the top right corner to show that the name is registered and that production is restricted to that pharmaceutical company as a sole owner.
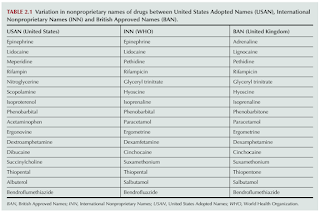
There is variation in nonproprietary names of drugs between United States Adopted Names (USAN), International Nonproprietary Names (INN) [World Health Organization] and British Approved Names (BAN).
- The existence of different approved names for the same drug molecule in different countries could lead to confusion and potentially serious medication errors.
Principles of Drug Nomenclature
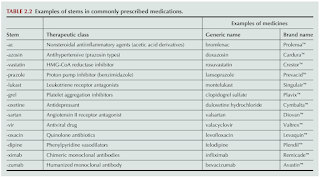
Currently, drug names are derived from a combination of a prefix, stem and suffix.
- Prefix
- is unique to each drug and does not relate to its structure or clinical function.
- In certain circumstances, prefixes such as "es-", "lev-" and "dex-" are used to describe stereoisomers of drugs.
- Inflix (sometimes)
- Stem or suffix
- Are used to indicate the pharmacological class of the drug.
Hence, understanding drug nomenclature can help us to predict drug classes and indications.
- There are exceptions to this rule, including nystatin, which is not a statin, and metronidazole, which is not an antifungal.
Also, the name should also be distinct in spelling and spelling to minimize the potential risks of confusion with another drug when misspelled or when handwritten.
Nomenclature of Biologics
The nomenclature of biotechnological products (biologics) has been quite dynamic and challenging because of complex nature of their chemical structure and diverse modes of pharmacological action.
- Hence, the international nonproprietary name could not be assigned using the general naming schemes and policies.
Comments
Post a Comment