HIV Infection
Introduction
HIV (Human immunodeficiency virus) is an enveloped, single-stranded RNA virus that was recognized as the causative agent of AIDS (acquired immune deficiency syndrome). If HIV is left untreated, the virus will cause progressive weakening of the immune system, a process which occurs at different rates in different people.
- 2 types of human HIV exist: HIV-1 and HIV-2. HIV-1 has worldwide distribution, whereas HIV-2 infection is endemic in West Africa.
NOTE: AIDS is diagnosed if the CD4 cell count is <200 or <14% of total lymphocyte in the presence of proven HIV infection, even in the absence of other infections.
Risk for HIV
HIV infection is usually acquired through sexual intercourse or exposure to infected blood or body fluids. This may occur
- During sexual contact with a person with HIV, especially if you have unprotected vaginal or anal sex.
- By sharing needles or syringes used by a person with HIV.
- People who received a blood transfusion or other blood products before 1984.
Transmission from a pregnant woman to her baby may occur during pregnancy, birth or breastfeeding, although this is uncommon with the use of HIV medications during and after pregnancy.
- Breast-feeding is not recommended as it is associated with risk of transmission up to 14% in those who are not virally suppressed. This risk reduces to 1% if the woman is virally suppressed.
NOTE: HIV infection is NOT spread by casual contact.
Treatment
Development of antiretroviral therapy (ART) have transformed HIV infection from an almost universally fatal illness to a chronic disease. However, ART cannot eradicate HIV from the human body nor cure HIV infection. Hence, it will be a life-long treatment.
Previous practice of ART initiation includes extensive patient preparation prior to starting ART. However, evidence from clinical trials have shown significant benefits of rapid ART initiation (within 7 days of diagnosis).
- Rapid ART initiation or same day ART initiation should be offered to all patients with HIV infection, regardless of CD4 count.
- Malaysian Consensus Guidelines on Antiretroviral Therapy generally recommends clinical assessment at the end of 2 weeks of opportunities infections (OI) therapy. If patient is stable and has improved with OI treatment, initiation of ART should not be delayed. In patients with OIs for which no effective treatment is available (cryptosporidiosis, microsporidiosis, progressive multifocal leukoencephalopathy and Kaposi’s sarcoma), ART itself can result in improvement and hence should be initiated as soon as possible.
Once ART is started, it should be taken for the rest of the life.
- An adherence to ART of 95% or more is believed to be the requirement to achieve optimal viral suppression.
- Taking ART drugs inconsistently (e.g. missing doses) can sometimes lead to development of viral resistance.
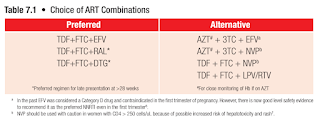
First Line ART
An antiretroviral regimen for a treatment-naive patient generally consists of 2 NRTIs (e.g. tenofovir, emtricitabine, abacavir, lamivudine, zidovudine) in combination with a third agent:
- Integrase inhibitor (e.g. dolutegravir, raltegravir)
- NNRTI (e.g. efavirenz, nevirapine)
- Protease inhibitor (e.g. lopinavir plus ritonavir, atazanavir plus ritonavir)
NOTE: Quadruple combination therapy has been found to be no more effective than triple combination therapy with currently available drugs.
At the end of year 2019, the Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV has updated its guidance to include a two-drug regimen (dolutegravir 50 mg plus lamivudine 300 mg OD) as an initial treatment option for most people with HIV infection, except for individuals with HIV RNA >500,000 copies/mL, hepatitis B coinfection, or in whom ART is to be started before the results of HIV genotypic resistance testing for reverse transcriptase or hepatitis B testing are available.
- The recommendation is based on two large, randomised controlled trials that showed that dolutegravir plus lamivudine was not inferior to dolutegravir plus tenofovir plus emtricitabine.
Therapy Goals
Treatment outcome may be measured from three aspects:
- Clinically by the reduction in the number and frequency of opportunistic infections (OIs) and improvement of general wellbeing
- Immunologically by gradual and steady rise in CD4 T-cell counts
- Virologically by a decrease in viral load (VL), ideally to undetectable level at six months after initiation of treatment (undetectable is defined as VL <20 copies/ml)
Treatment failure
HIV viral load is more accurate and reliable than CD4 cell counts to monitor treatment response and for early detection of treatment failure.
- Effective therapy should generally result in a 10-fold (1.0 log10) decrease in HIV-1 RNA copies/ml in the first month and suppression to less than 200 copies/ml by 6 months.
Viral "Blips"
- Defined as isolated transient rises in viral load to above detected level while on treatment after having achieved prior viral suppression and is followed by re-suppression.
- The levels generally do not exceed 200 copies/ml.
- It may reflect technical variations in laboratory assay performance, or biological events associated with viral replication.
- Isolated "blips" are not associated with subsequent virologic failure.
Low Level Viremia
- Defined as persistently low, but detectable viral load in the range of 50-1000 copies/ml.
- Evidence shows that incomplete viral suppression leads to the accumulation of resistance, mutations with a concomitant increase in viral replication, reduction in CD4 cell counts, increased risk of virologic progression and clinical deterioration.
Virologic Failure
- The optimal threshold for defining viral failure and for switching ART regimens has not been established.
- WHO defines virologic failure by a persistently detectable viral load exceeding 1000 copies/ml (that is, two consecutive viral load measurements within 3-month interval with adherence support between measurements) after at least 6 months of starting a new ART regimen.
Post-Exposure Prophylaxis
All efforts must be made to initiate PEP as soon as possible, preferably within 2 hours of exposure.
- 2-drugs-regimen for HIV positive source patient already on HIV treatment and recent viral load is undetectable.
- Preferred: Tenofovir 300 mg OD and Emtricitabine 200 mg OD.
- Alternative: Zidovudine 300 mg BD and Lamivudine 150 mg BD.
- Add for 3-drugs-regimen for HIV positive source patient not on treatment or on HIV treatment but recent viral load is still detectable or no recent viral load.
- Preferred: Dolutegravir 50 mg OD or Raltegravir 400 mg BD.
- Alternative: Lopinavir/ritonavir 250 mg 2 tablets BD.
NOTE: Animal studies have shown that PEP is most likely to be effective when initiated within 24-36 hours.
If source is unknown (e.g. pricked by a needle in a general waste bin), the decision to give PEP should be individualized depending on HIV risk profile of the patients in the area in which the needle was found and the likelihood of the sharp having been used recently.
Duration of PEP is 28 days. Emphasis on adherence to treatment and completion of the course is important to achieve PEP effectiveness.
HIV testing should be repeated at 4 weeks, and 12 weeks after exposure. It is recommended that other blood borne diseases such as Hepatitis B and C screening also be repeated at the same time.
- During the 12 week follow up period, HIV-exposed HCWs should be advised to use condoms to prevent potential sexual transmission; avoid pregnancy and breast feeding in female HCWs; and refrain from donating blood, plasma, organs, tissue or semen.
Antiretroviral Therapy Among Serodiscordant Couples
A serodiscordant couple, also known as mixed-status, is when one partner is HIV-negative and the other is HIV-positive.
Treatment as Prevention (TasP)
- People living with HIV should take ART to treat HIV as soon as possible to improve their own health and prevent transmitting HIV to their sexual partner(s).
- Undetectable=Untransmittable (U=U) concept: A person living with HIV who has undetectable viral load does not transmit their HIV to their partners.
Couples with differing HIV status who want to conceive
Safer conception strategies include
- ART for HIV-positive partners. It is important that they remain adherent to ART and achieved sustained viral suppression (2 recorded HIV viral load result which were below detectable levels at least 3 months apart) before attempting conception.
- Pre-exposure prophylaxis (PrEP) for HIV-negative partners in situation where their HIV-positive partners have not achieved viral suppression yet or viral suppression status are not known.
- Assisted reproductive technologies like artificial insemination.
- Both partners should be screened and treated for any sexually transmitted infections before attempting to conceive.
Prevention of Mother-to-Child Transmission
Antenatal combination antiretroviral therapy (ART) is the recommended for prevention of mother-to-child transmission (PMTCT).
- For women who are stable on ART before pregnancy, the existing ART is to be continued throughout pregnancy and after delivery, unless taking a regimen that is contraindicated in pregnancy.
- Achieving HIV viral suppression by the third trimester reduces the risk of transmission to 0 to <0.5%.
Women who present after the second or third trimester must commence ART without delay.
A viral load must be between weeks 32-36 to determine ongoing risk of transmission to the foetus and to determine mode of delivery [spontaneous vaginal delivery (SVD) or pre-labour elective caesarean section (PLCS)].
- In women who had not achieve maximal viral load suppression, PLCS has been proven to further reduce the risk of transmission.
Intrapartum intravenous zidovudine infusion (2 mg/kg for the 1st hour followed by 1 mg/kg/h subsequently) is recommended for women with a viral load of >1000 copies/ml, who present in labour or with ruptured membranes or who are admitted for planned PLCS.
- For PLCS, intrapartum IV zidovudine should be started 3 hours before surgery.
- Current evidence suggests that intrapartum IV zidovudine has no additional benefit in prevention of vertical transmission in pregnant women on ART with viral load ≤1000 copies/ml during late pregnancy and near delivery.
Breastfeeding is not recommended as it is associated with risk of transmission up to 14% in those who are not virally suppressed.
- The risk reduces to 1% if the woman is virally suppressed.
- However, breastfeeding is still not encouraged in our population.
- For women on ART, compliance must be stressed if they insist on breastfeeding their baby.
Management of babies born to HIV infected mother
- Children born to HIV positive mothers are usually asymptomatic at birth. However, all will have acquired maternal antibodies.
- In uninfected children, antibody testing becomes negative by 10-18 months age.




Comments
Post a Comment