Antiretroviral Therapy
Introduction
An antiretroviral regimen for a HIV treatment-naive patient generally consists of 2 NRTIs in combination with a third agent:
- Integrase inhibitor (INSTI)
- NNRTI
- Protease inhibitor (PI)
The choice between an INSTI, PI, or NNRTI as the third drug in an initial ARV regimen should be guided by the regimen’s efficacy, barrier to resistance, adverse effects profile, convenience, comorbidities, concomitant medications, the potential for drug-drug interactions and the availability.
NOTE: If drugs are used individually, resistance rapidly develops, so antiretroviral therapy is given as a combination of drugs.
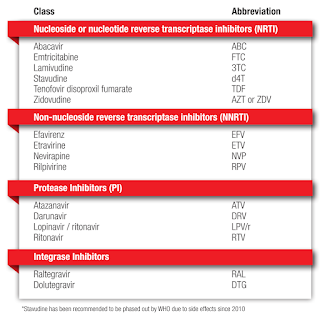
Nucleoside or Nucleotide Reverse Transcriptase Inhibitor (NRTI)
Tenofovir- Tenofovir and zidovudine are generally comparable in terms of efficacy; however, some studies have shown better efficacy and less side effects with tenofovir-based therapy compared to zidovudine.
- Tenofovir Alafenamide (TAF) and Tenofovir Disoproxil Fumarate (TDF) are two approved forms of tenofovir and can both be used in patients with Hepatitis B coinfection.
- TDF has been associated with bone and kidney toxicities (should be avoided in patients with chronic kidney disease with CrCl <50 ml/min), especially when used with Ritonavir. TAF is a prodrug of tenofovir that yields relatively lower plasma levels and high intracellular levels of tenofovir and is less likely to cause kidney and bone toxicities than TDF. However, TAF should not be coadministered with rifamycins as it would reduce the drug levels. Conversely, levels of fasting low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, and triglycerides increased more in the TAF group than in the TDF group with no change in total cholesterol to HDL ratio.
- Zidovudine should be discontinued if Hb has dropped ≥25% of baseline or < 8.0 g/dL.
- Lipoatropy (fat wasting) at face, arms, leg and buttocks is associated with history of exposure to stavudine or zidovudine.
- Lactic acidosis with hepatic steatosis (rare but potentially life-threatening toxicity) is reported.
- Abacavir is not recommended in cases where HIV viral is >100,000 copies/ml unless third agent is dolutegravir or protease inhibitor.
- Abacavir hypersensitivity occurs in at least 5% of patients and is associated with HLA-B*5701 allele.
- Several observational studies linked abacavir use with cardiovascular disease and cardiac events (e.g. myocardial infarction, ischaemic stroke).
Non-nucleoside reverse transcriptase inhibitors (NNRTI)
NNRTI has low genetic barrier to resistance with long half-lives. Abrupt discontinuation of NNRTI without maintaining NRTIs backbone will increase the risk of NNRTI resistance due to its long half-life. Hence, when NNRTI is stopped due to adverse event, the backbone NRTIs should be continued for at least 1 week before stopping all drugs.
Nevirapine- Nevirapine and efavirenz have comparable clinical efficacy when used in combination ART. However, nevirapine is associated with higher risk of rash, Steven-Johnson Syndrome and hepatotoxicity compared to efavirenz.
- NVP must be avoided in women with CD4 count >250 cells/mm3 and men with baseline CD count >400 cells/mm3 due to significant increase in incidence of symptomatic hepatic events.
- Lead in dosing of 2 weeks for nevirapine should be practiced to decrease risk of hepatitis and rash.
- CNS adverse effects of efavirenz include tiredness, dizziness, impaired concentration, drowsiness, insomnia and abnormal dreaming, occur in 50% of patients and generally resolve after 2-4 weeks, but may persist long-term. Avoid heavy or oily food to reduce symptoms.
- Efavirenz should be avoided in patients with severe psychiatric illness and in those whose daily functional status is affected by its side effects.
- In the ENCORE 1, efavirenz 400 mg daily was found to be better tolerated than standard of efavirenz 600 mg daily with comparable viral suppression.
- Efavirenz hypersensitivity reactions may be accompanied by elevated liver transaminases.
Rilpivirine
- Rilpivirine is recommended in individuals who have baseline viral load of less than 100,000 copies/ml.
- It needs to be taken with food and has significant interactions with acid reducing agents; Proton pump inhibitors are contraindicated.
Integrase Inhibitors (INSTI)
Integrase strand transfer inhibitors has emerged as a recommended first line regimen (in combination with 2 NRTIs) for most people with HIV infection due to higher rates of viral suppression, favourable adverse effects profile and the tolerability of the medication.
- Advantages
- Higher and more rapid rates of viral suppression
- Lower potential for drug-drug interaction
- Higher genetic barrier for HIV drug resistance
- The WHO recommends dolutegravir over a boosted protease inhibitor for second line therapy. The DAWNING study showed superiority of dolutegravir over ritonavir-boosted lopinavir in second line treatment.
- The risk of neural tube defects associated with using dolutegravir at conception has declined since the initial report released in May 2018. As more data has been added to the Tsepamo Cohort, the difference in dolutegravir vs non dolutegravir regimens regarding risk of neural tube defects is now no longer statistically significant.
- Adverse events: Insomnia (≤7%), hepatotoxicity, hyperglycemia (≤9%) and hypersensitvity syndrome (<1%)
Raltegravir
- The first INSTI approved for use in both treatment naive and treatment experienced patients.
- It has a relatively low barrier to resistance.
- Adverse events: Increased serum alanine aminotransferase, increase in fasting glucose, DRESS syndrome (<2%)
Protease Inhibitors (PI)
Protease inhibitors such as atazanavir/ritonavir may be considered as the third agent in a first line ART regimen if the patient has the following clinical scenarios.
- Unable to tolerate the side effects of integrase inhibitors/NNRTI.
- Baseline resistant testing indicating a resistance to standard first line agent
Atazanavir
- Asymptomatic elevations in bilirubin occur commonly during atazanavir therapy and are reversible upon discontinuation of treatment. Consider alternative therapy if jaundice or cosmetic concerns.
Darunavir
- Skin rash (10%) - Darunavir has a sulphonamide moiety; Stevens-Johnson syndrome and erythema multiforme have been reported.
Ritonavir (Booster drug)
- Since it inhibits the CYP 450 liver enzymes that metabolise protease inhibitors, drug interactions should be checked via the University of Liverpool's HIV Drug Interactions website before prescribing a new drug for the patient.
External Links
- Malaysian Consensus Guidelines on Antiretroviral Therapy, 2022
- Prescribing for patients taking antiretroviral therapy, 2022
- Efficacy of 400 mg efavirenz versus standard 600 mg dose in HIV-infected, antiretroviral-naive adults (ENCORE1): a randomised, double-blind, placebo-controlled, non-inferiority trial, 2014
- Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): an open-label, non-inferiority, phase 3b trial, 2019
- Update on neural tube defects with antiretoviral exposure in the Tsepamo study, Botswana, 2021
Hey! I have been reading your posts for a very long while and would like to drop by and say a big THANK YOU!
ReplyDeleteIt's really inspiring to see such a committed person in sharing your wealth of knowledge and helping others :)
Really appreciate your selfless contribution <3
Please keep up the good work! My best wishes for you :D
Thanks for the encouraging words. It is glad to hear that you find the information to be useful. Share it with your friends or colleagues.
DeleteYeah me too frequent visitor.thanks alot
Delete