Basic Pharmacokinetics
Introduction
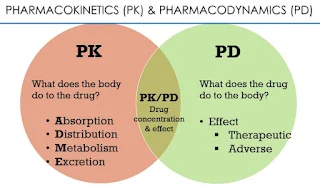
Pharmacokinetics - The study of the time course of drug absorption, distribution, metabolism and excretion (ADME).
Pharmacodynamics - The relationship between drug concentration at the site of action and resulting effect, including the time course and intensity of therapeutic and adverse effects.
Absorption
With intravenous route, all of the administered drug is absorbed. However, if a drug is administered by any other route, it must be absorbed into the blood stream. This process may or may not be 100% efficient.
Bioavailability (F) is the fraction of the administered dose which is absorbed into the bloodstream.
- It only estimates the extent of absorption, not take into consideration the rate of drug absorption.
Factors that affect bioavailability include
- Inherent dissolution and absorption characteristics of the administered chemical form (e.g. salt, ester)
- The dosage form (e.g. tablet, capsule)
- The route of administration
- The stability of the active ingredient in the gastrointestinal tract
- The extent of drug metabolism before reaching the systemic circulation
Distribution
Subjected to physical (such as molecular size or weight) or chemical (such as lipophilicity/hydrophilicity or ionization) properties, drugs may be distributed in different concentrations in various parts of the body.
Volume of distribution (Vd) is theoretical size of the compartment necessary to account for the total drug amount in the body if it were present throughout the body in the same concentration found in the plasma.
- In other words, a large volume of distribution usually indicates that the drug distributes extensively into body tissues and fluids.
- Conversely, a small volume of distribution often indicates limited drug distribution
The apparent volume of distribution may be used to determine the plasma concentration after an intravenous loading dose.
Plasma Concentration Estimated = Dose / Vd
After considering bioavailability (F) and salt factor (fraction of active drug when the dose is administered as a salt) (S) for non-IV routes,
Plasma Concentration Estimated = S * F * Dose / Vd
Elimination
Drugs may be eliminated from the body by a number of routes.
- Excretion of the unchanged drug in the kidneys
- Metabolism (usually in the liver) into a more water-soluble compound for subsequent excretion in the kidneys
- Combination of both
Clearance (CL) is defined as the volume of plasma completely emptied of drug per unit time.
CL = CLmetabolic + CLrenal
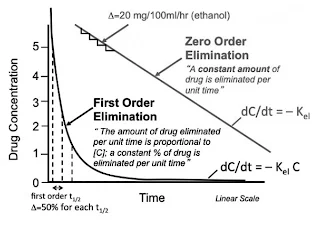
For drugs undergoing first-order elimination, there are elimination rate constant (ke) and elimination half-life (t1/2).
CL = ke * Vd
t1/2 = ln 2 / ke
When the rate of administration is equal to the rate of elimination, the drug is said to achieve steady state concentration.
- In the absence of a loading dose, approximately 5 half-lives are required to achieve steady state.
The elimination half-life (t1/2) represents the time required for a drug's plasma concentration to decrease by 50%.
- After approximately five half-lives, the concentration will be negligible.
- Consequently, drugs with short half-lives often necessitate more frequent dosing to maintain therapeutic levels.
Comments
Post a Comment