Transdermal Patches
Introduction
Prefabricated transdermal patches are designed to deliver a constant and controlled dosage over extended periods for systemic therapy.
They offer advantages over conventional oral dosage forms in that
- Drug administration through the skin avoids the pH variations seen with gastrointestinal transit.
- The drug reaches the systemic circulation whilst avoiding first-pass hepatic metabolism (though the skin has some metabolic activity).
- Patches can be removed easily and quickly in cases where adverse drug reactions occur.
- Patient adherence is high.
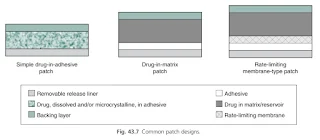
Designs of Transdermal Patches
The simplest transdermal systems contain the drug in an adhesive, with more complexity introduced in matrix-type patches and reservoir systems.
Simple drug-in-adhesive patch
- These patches are formed by dissolving or dispersing drug within an adhesive, which is then coated onto a backing layer before a release liner is applied.
- Different daily dosages can easily be delivered by increasing the surface area of the patch; a 20 cm2 patch will deliver double the daily dose of an equivalent 10 cm2 patch.
- Patches are typically intended to deliver the drug at a constant rate for the duration of use e i.e. zero order. In practice, for a patch where the drug is dissolved in an adhesive, the concentration of the drug in the patch drops as molecules leave and enter the skin. The reduction in the concentration gradient from the patch to the skin thus slows delivery, although initially to only a marginal extent. To mitigate against this, the adhesive layer can be made thicker and hence contains a greater drug loading so release of a given amount of drug is less marked, or additives can be added to the adhesive to increase drug solubility, in addition to excipients that may aid delivery or enhance drug stability.
Drug-in-matrix patch
- A drug matrix or reservoir is usually prepared by dissolving the drug and polymers in a common solvent before adding in other excipients such as plasticizers.
- The viscosity of the matrix can be modified by the amounts of polymers incorporated, or by cross-linking polymers in the matrix, and can consequently be used to control diffusion of the active ingredient through the matrix to the adhesive and then on to the skin surface.
- Reservoirs may use a viscous liquid, such as a silicone or a cosolvent system, occasionally with ethanol, into which the drug is dissolved and dispersed. In these cases, drug diffusion within the reservoir towards the skin surface is unhindered.
Rate-limiting membrane-type patch
- In practice, it is usually the stratum corneum barrier that limits the rate of drug input into the skin and hence provides the rate limiting barrier.
- However, semipermeable membranes are used to separate reservoirs from the underlying adhesive and can also be found separating multiple drug-in-adhesive layers.
- Various polymers can be used for such membranes, including poly(ethylene-vinyl acetate), with or without plasticizers.
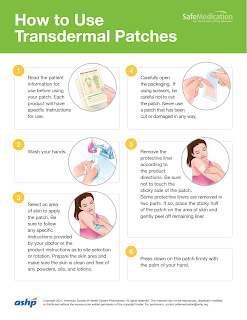
Transdermal Patch Application
Each product will have specific instructions for use. Common application sites are
- Chest (upper)
- Back (upper and lower)
- Upper arm (on the part facing out)
- Flanks (sides of the body, abdomen level)
NOTE: The upper back may be preferable in a confused person, to reduce the risk of unintended patch removal.
Make sure the skin area for patch application is clean, hairless and free from dirt, lotions, oils and powder.
- The skin should not be shaved shortly before applying; shaving is irritating to the skin. If needed, cut the hair short with scissors.
Carefully open the packaging and remove the protective liner.
- Be sure not to touch the sticky side of the patch.
- Some protective liners are removed in two parts. If so, place the sticky half of the patch on the area of skin and gently peel off remaining liner.
Press the patch firmly onto the chosen skin site with the palm of your hand and hold there for at least 10-30 seconds.
- Make sure it sticks well, especially the edges.
Remember to rotate the location where you apply your patch to prevent irritation.
Avoid heat exposure with most patches because heat causes rapid absorption of the medication from the patch, resulting in toxicity.
- E.g. Not to apply a patch immediately after a person has had a bath or shower.
Replace patches according to leaflet suggestion
- Buprenorphine patch every 7 days
- Fentanyl patch every 3 days
- Ketoprofen patch every 12 hours
- Rivastigmine patch every day
In most cases, remove and fold the patch to press adhesive surfaces together for disposal.
- Remember used patches can contain residual medicine which can be fatal.
Cutting Patches
Avoid cutting patches if possible:
- Cutting a patch will make the use ‘off-licence’.
- The release mechanism of the medicine could be adversely affected and release more medicine than intended.
- Cutting patches with a rate-limiting membrane can cause the medicine to leak from the patch and is therefore not recommended.
- The matrix type patches can be sometimes cut because the medicine is embedded in an adhesive matrix with the release rate determined by the physical properties of the matrix.
- The resulting sharp edges could impair adhesion to the skin.
- Surgical adhesive tape (e.g. Micropore) can be applied to the edges to aid adherence.
External Links
- SafeMedication - How to Use Transdermal Patches
- Transdermal patches: history, development and pharmacology, 2015
- SPS - Using transdermal patches safely in healthcare settings
- Transdermal fentanyl patches: life-threatening and fatal opioid toxicity from accidental exposure, particularly in children, 2018

Comments
Post a Comment