Insulins
Introduction
Insulin is used in the treatment of diabetes mellitus.
- Generally, all people with type 1 diabetes mellitus need insulin treatment; many individuals with type 2 diabetes will require insulin as their beta cell function declines over time.
Indications
Insulin therapy should be considered in the following conditions.
- Type 1 diabetes.
- Inadequate glycemic control on optimal dose and number of oral glucose lowering drugs in Type 2 diabetes.
- Severe hyperglycemia on presentation (e.g. HbA1c >10% or fasting plasma glucose >13.0 mmol/L).
- As part of an insulinisation treatment regimen in type 2 diabetes mellitus
- Short-term use in acute illness or surgery, pregnancy, breast-feeding and severe metabolic decompensation (e.g. diabetic ketoacidosis).
- Difficulty distinguishing types of diabetes.
- Although the peak incidence of type 1 diabetes occurs around the time of puberty, approximately 42% of cases present after 30 years of age.
NOTE: Timely initiation of insulin in individuals with T2DM has been associated with multiple benefits, such as decreasing the glucotoxic effects of hyperglycemia, preserving beta-cell mass/function, improving insulin sensitivity and long-term protection from chronic complications.
Types of Insulins
Insulin formulations have advanced from its beginnings of using animal sources (e.g. porcine and bovine sources) to human insulins engineered using DNA recombinant technology followed by insulin analogues that closely mimic human physiologic insulin. More recently, biosimilar insulins have been introduced to the market.
- Human insulin derived by recombinant technology
- After regular insulin (short-acting) is injected subcutaneously, the hexamers that have formed dissociate into dimers and monomers that are absorbed. This causes a delay in rise of insulin concentrations in the blood stream, resulting in a need to inject at least 30 minutes before the meal to best cover post-meal glycemic excursions.
- Neutral protamine Hagedorn (NPH) insulin is a crystallized suspension of human insulin, protamine and zinc in a neutral buffer that delays the release of the insulin into the bloodstream (intermediate-acting).
- Basal intermediate acting insulin should be administered pre-bed (preferably not earlier than 10 pm) because of the risk of hypoglycemia in the early hours of the morning if given earlier.
- Insulin analogues which are genetically modified human insulin.
- Rapid-acting insulin analogues (e.g. insulin lispro, lispro-aabc, aspart, faster aspart and glulisine) have faster onset and shorter duration of action than regular insulin for pre-meal coverage.
- Long-acting analogues (e.g. insulin glargine, determine, degludec) have a longer, flatter and more predictable day-to-day profile than NPH for basal coverage (i.e. lower hypoglycemic episodes).
Based on their pharmacokinetic profiles, types of insulin are
- Prandial (bolus)
- Rapid or short-acting insulin.
- To cover the extra requirements after food is absorbed.
- Basal
- Intermediate or long-acting insulin.
- To suppress hepatic glucose production and maintain glucose levels at target in the fasting state.
- Premixed
- Biphasic insulin that incorporates both the short or rapid-acting insulin with intermediate or long-acting insulin in a single preparation.
- Should always be dosed before meals because the fast-acting component is meant to cover prandial intake.
- To limit the number of daily injections.
- Co-formulations
- A combination of two types of insulin or an insulin with a glucagon-like peptide-1 receptor analogue (GLP1-RA).
Disadvantages
The major drawbacks associated with insulin therapy are
- Weight gain
- Hypoglycemia
Glycemic Control Targets
Fasting or pre-prandial
- Outpatient: 4.4-7 mmol/L
- General ward or ICU admission: 7.8-10 mmol/L. Initiate insulin therapy if blood glucose >10 mmol/L.
Post-prandial (at least 90 minutes after meals)
- 4.4-8.5 mmol/L
- Insulin should be initiated once blood glucose persistently ≥10 mmol/L.
- Once insulin therapy is started, a target glucose range of 7.8-10.0 mmol/L is recommended for the majority of critically ill and non-critically ill patients.
- In selected patients, such as cardiac surgery patients, and patients with acute ischaemic cardiac or neurological events, lower glycaemic targets may be recommended as long as this can be achieved without significant hypoglycaemia.
- Patients with a prior history of successful tight glycaemic control in the outpatient setting who are clinically stable may be maintained with a glucose target below 7.8 mmol/L.
- Higher blood glucose ranges may be acceptable in terminally ill patients, in patients with severe comorbidities, and in in-patient care settings where frequent glucose monitoring or close nursing supervision is not feasible.
- <7% for most
- ≤6.5% for younger age and healthier patients
- 7.1-8% for elderly patients and patients with co-morbidities (e.g. advanced CVD, heart failure, dementia, advanced renal failure)
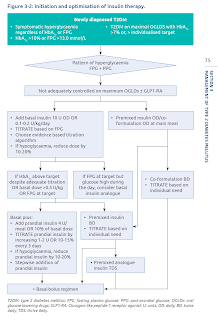
Insulin Initiation and Optimisation in Type 2 Diabetes Mellitus
An ideal insulin regimen should mimic the physiological insulin response to meals and endogenous hepatic glucose production.
- The choice of insulin regimen should be individualized, based on the patient's glycemic profile, dietary pattern, personal lifestyle and desired flexibility.
Initiate basal insulin first.
- In addition to oral and non-insulin injectable agents.
- Initial dose: 0.1-0.2 IU per kg (minimum 10 IU, up to 15 to 20 IU) daily.
- For patients with a risk of hypoglycaemia, such as the elderly, patients who are lean or those with milder (lower) fasting hyperglycaemia, a lower starting dose should be considered
- If fasting glucose levels are very elevated (>13.9 mmol/L), A1C is >8 percent, or if a patient is known to be very insulin resistant, initial doses of basal insulin can be higher (e.g., 0.3 IU per kg or up to 15 to 20 IU daily as an initial dose).
Titrating dose.
- Dose adjustments should be done after 3-7 days of initiating the last dose, with at least 3 consecutive readings, to achieve the individualized target fasting plasma glucose (FPG) without hypoglycaemia.
- Set fasting blood glucose target (e.g. 4.4-7 mmol/L) and increase/decrease the dose by 2-4 IU or 10-20% every 3 days.
- Titration can be more aggressive (e.g. 5-6 IU every 3 days) if fasting glucose levels are very elevated (>13.9 mmol/L) or if a patient is known to be very insulin resistant.
- Basal insulin has a "ceiling effect", i.e., fasting plasma glucose (FPG) reductions become proportionally smaller with increasing doses. The "ceiling effect" has been shown to occur at a basal insulin dose of 0.5 U/kg/day (range 0.3 U/kg/day to 1 U/kg/day).
Alternatively, convert to premixed insulin once or twice daily.
- The major drawback of premixed insulin is limited flexibility in adjusting doses.
- However, premixed insulin is a reasonable option for patients with type 2 diabetes who are doing well on a stable, fixed ratio of short- and intermediate-acting insulin, or who are able to modify their diets to match the kinetics of premixed insulin.
- Premixed insulin works best when there is little day-to-day variability in breakfast, lunch, and dinner (with a small lunch) or when people are so insulin resistant that they are unlikely to develop hypoglycemia after smaller meals.
- To initiate, calculate the total daily dose based on weight (0.2 IU per kg [minimum 10 IU, up to 15 to 20 IU] daily) or based on prior insulin dose.
- For twice daily dosing, premixed insulin should be dosed relative to meal intake and may need to be reduced for smaller meals.
- Approximately 50-70% of the total daily dose is administered in the morning and 50-30% at dinner.
- Pre-breakfast plasma glucose determines pre-dinner premixed dose adjustment, while pre-dinner plasma glucose determines pre-breakfast premixed dose.
- Adjust insulin doses after 3 consecutive plasma glucose values obtained (every 3-7 days) by increasing or reducing dose by 2 IU or 10-15%.
Initiate prandial insulin if persistent elevation in HbA1c with fasting glucose in target range.
- If indicated, prandial insulin of approximately 4-6 IU, 10% of basal insulin dose or 0.1 IU/kg is often started as a single injection before the largest meal of the day, but many strategies are possible.
- The prandial insulin dose can be increased by 1-2 IU or 10-15% every 3 days until the postprandial blood glucose target is achieved.
- Prandial insulin dosage adjustments depend on the current dose. As a rule of thumb for current pre-meal dose:
- ≤10 IU - Adjust in 1-unit increments
- 11 to 20 IU - Adjust in 2-unit increments
- >20 IU - Adjust in 5-unit increments (or more, depending on patient insulin resistance and meal size and content)
- A more complex method for adjusting pre-meal insulin is to match insulin delivery to the anticipated glucose excursion with meals. Many patients benefit from specific training in carbohydrate counting, which requires some arithmetical computations that some patients find difficult or burdensome.
- If hypoglycaemia occurs, identify the cause. If there is no cause, reduce the bolus insulin by 2-4 IU or 10-20% of the basal dose.
- Check fingerstick capillary glucose levels fasting, pre-lunch, pre-dinner before bed to further adjust insulin regimen.
- Intensifying the basal-plus regimen by the sequential addition of bolus insulin, will ultimately lead to a basal-bolus regimen.
- The ability to inject rapid-acting insulins 10 to 15 minutes before meals (as opposed to the 30 to 45 minutes before the meal with regular [short-acting] insulin) is more convenient and may improve adherence.
NOTE: Physiologic replacement of insulin with "basal-bolus" insulin therapy should be started as early as possible following the diagnosis of type 1 diabetes.
General Guidelines for Long Term Use of Insulin
Requirements of high dose of insulin (total daily dose >1.5-2 IU/kg) should prompt a search for an underlying cause such as non-adherence or incorrect injection technique.
In general, total daily dose of prandial insulin should not be >50% of total daily dose.
Compared with intermediate-acting insulins, longer-acting insulins may have less glucose-lowering effect overnight and more effect into the next day.
- Accordingly, when switching between basal insulins, UpToDate generally reduce the dose by 10-20% to decrease the risk of hypoglycemia, then increase the dose as needed to maintain glycemic targets.
- In patients with marked hyperglycemia, the equivalent total daily dose may be used.
Sulfonylureas or meglitinides should be discontinued once bolus insulin is used regularly with meals.
Monitor weight gain.
Read up insulin injection techniques at a separate post.
External Links
- UpToDate - General Principles of Insulin Therapy in Diabetes Mellitus
- UpToDate - Insulin Therapy in Type 2 Diabetes Mellitus
- Management of Type 2 Diabetes Mellitus, 2020
- Practical Guide to Inpatient Glycemic Care, 2020
- Practical Guide To Insulin Therapy in Type 2 Diabetes Mellitus, 2024
- DMTAC Pocket Guide to Insulin Optimisation, 2023




This explanation about how insulin breaks down before absorption really helped me understand why timing is so important with subcutaneous injection services. It’s interesting how the body handles insulin in stages, which makes the 30-minute wait before meals make a lot of sense. It’s clear that good guidance on injection timing can really improve blood sugar control.
ReplyDelete