pH and pKa
Introduction
Functional groups that are either acidic or basic have the ability to become ionized and as a result become negatively or positively charged, respectively, within the structure of a drug molecule.
- Acidic functional groups are those can donate (or lose) a proton (H+) and the resulting atoms must be able to delocalize the resulting negative charge via resonance.
- Basis functional groups are those that can accept (or gain) a proton (H+). It must have a nitrogen atom with a lone pair of electrons that can bind to and accept a proton.
This ability of ionize increases the overall water solubility of the drug molecule, allows for the formation of specific types of interactions between the drug molecule and its biological target(s), and influences the transport, metabolism and elimination of the drug molecule.
- The extent to which a functional group can ionize depends on its pKa and the environment in which it resides.
- For example, acids are primarily unionized in an acidic environment, while bases are primarily ionized in an acidic environment. The opposite is true in a basic environment.
Defining pH and pKa
By definition, pH is equal to the negative log of the hydrogen ion concentration in a solution.
pH = - log[H+]
- The pH of a solution can change based on what is added or removed from the solution.
- Low pH values indicate acidic environments, while high pH values indicate basic environments. A pH at or about 7.0 indicates a neutral environment.
By definition, pKa is equal to the negative log of the Ka, the dissociation constant for an acid in an aqueous environment.
- Low pKa values indicate either strongly acidic functional groups or weakly basic functional groups.
- High pKa values indicate either weakly acidic functional groups or strongly basic functional groups.
Henderson-Hasselbalch Equation
The Henderson-Hasselbalch equation provides a mathematical relationship among the pKa value of a functional group, the pH of the environment in which it resides and the ratio that exists between the ionized and the unionized form of the functional group.
Key summary points
- First, you have to identify the acidic and basic functional group(s) and correctly assign the given pKa value(s).
- When the pH equals the pKa, the functional group is 50% ionized 50% unionized.
- When the pH is greater than the pKa, acidic functional groups are primarily ionized and basic functional groups are primarily unionized.
- When the pH is less than the pKa, acidic functional groups are primarily unionized and basic functional groups are primarily ionized.
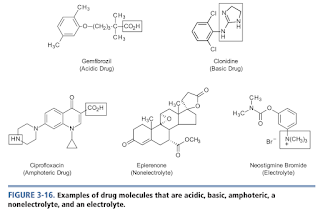
Functional Groups Versus Drug Molecules
Any given drug molecule may contain one or more of functional groups within its structure.
- Acidic drugs - contain one or more acidic functional groups and no basic functional group within its structure.
- Basic drugs - contain one or more basic functional groups and no acidic functional groups within its structure.
- Amphoteric molecules - Contain at least one acidic and one basic functional group.
- Nonelectrolytes - Contain only neutral functional group.
- Electrolytes - contain a quaternary ammonium salt and no other ionizable functional groups (and therefore are unable to accept or donate a proton).
Summary
A comprehensive understanding of pH and pKa is fundamental in drug design. These physicochemical properties significantly influence a drug's behavior within the human body.
- The ionization state of a drug, governed by its pKa and the surrounding physiological pH, critically impacts its solubility, absorption, distribution, and ultimately, its interaction with biological targets.
- Importantly, drugs tend to dissolve more readily in the ionized phase but more easily pass through plasma membranes in the non-ionized form, facilitating faster absorption.
- Moreover, pH and pKa play a crucial role in drug formulation, stability, metabolism, and renal excretion.
- Notably, site-specific drug delivery can be pH-dependent.




nice
ReplyDeleteThis blog post offers a comprehensive overview of the relationship between pH and pKa, emphasizing their significance in pharmacology. The author effectively explains how the ionization state of drug molecules, influenced by pH and pKa, impacts their solubility, absorption, and distribution within the body. The inclusion of functional group classifications acidic, basic, amphoteric, and nonelectrolytes—provides clarity on how different drugs behave in various pH environments.
ReplyDelete