Eye Preparations
Introduction
Physiological and biochemical exists to protect the eye from harmful stimuli.
- Tears contain lysozymes and immunoglobulins, which impart an anti-infectious activity.
- While these mechanisms are protective, they do sometimes present to drug absorption.
- The tear volume in the normal is 5-9 µL.
- Basal tears are continuously secreted by the lacrimal glands at an average rate of 1.2 µL/min, thus giving a tear turnover rate of approximately 17% per minute.
- Reflex tears are triggered by irritants, and their secretion rate ranges from 3 to 400 µL/min, the intention being rapidly to eliminate the stimulus.
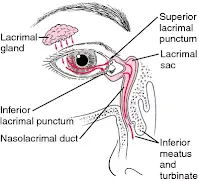
- Another protective mechanism is the eyelid movements associated with blinking.
- Blinking moves tear fluids and foreign matter to the nasal corner of the lid surface, where the liquid exits via the puncta and is then drained away by the nasolacrimal ducts into the inferior nasal passage - often called the lacrimal pump
Topical ophthalmic preparations are medications applied directly to the eye to treat a variety of conditions, such as dry eyes, allergies, glaucoma, infections and inflammation.
- It is important to use them correctly to achieve maximum efficacy.
Eye Drops
The combined mechanisms of lacrimal drainage and blinking mean that administered eye drops are rapidly cleared, with residence times ranging from 4 to 23 minutes.
- This helps to prevent the drops from staying in the eye for too long and causing side effects.
Moreover, the conjunctival sacs can only accommodate 20 to 30 µL of added fluid without spilling; however, the typical drop volume from eye drop bottles made by different manufacturers ranges from 34 to 63 µL.
- In other words, one eye drop volume exceeds the volume that can be accommodated by the eye.
As a result,
- There is not often a need to administer more than one drop at once.
- Administered eye drops should be spaced by at least 5 minutes ideally to minimize washout.
- Punctual occlusion by the closing of the eye and gently pressing of the inside corner for at least 1 minute maximizes local absorption and minimizes systemic exposure by up to 70%.
Plus, it is helpful to counsel patients to look upward when others are helping them to administer their eye drops.
Eye Drops in Children
Most eye drops administration counselling that we have read is targeted on adults, but how about neonates who don't even understand the need of him to have an eye drop? Are there any differences?
Australian Medicines Handbook 2021 suggests:
- Hold the child’s eyelids open between the index finger and thumb of one hand and put drops in with the other. If this is difficult, put a couple of drops onto the skin at the inner corner of the eye and wait for the eyes to open.
- Infants and toddlers may need to be held still during administration. If you don’t have someone to help you may need to swaddle the child with a sheet or lay them on the floor and gently hold their head still between your knees.
How Many Drops are in 1 ml of Eye Drops?
When calculating the number of eye drop bottles that a patient needs each month, it is often assumed that 1 ml equals to 20 drops.
- However, this assumption is not always accurate.
- The eye drops size are affected by factors such as the design and physical characteristics of the dropper tip and bottle, the physio-chemical properties of the solution (e.g. viscosity), and the manner in which the patient dispenses the drops (e.g. horizontal, 45 degree angle and vertical holding).
- For example, a 10 ml Hylo-Comod provides 300 drops and each Xalatan eye drop bottle contains 2.5 ml eye drops corresponding to approximately 80 drops of solution.
In 2017, Moore et al. looks specifically into the variability in number of drops per bottle of glaucoma medication.
- A total of 192 bottles from 32 bottle designs and manufacturers were tested.
- 22 of the 32 bottle designs had a significantly different mean number of drops in the vertical and horizontal positions, with 10 designs have more drops dispensed in the horizontal orientation and 12 in the vertical orientation.
- 6 of the 32 bottle designs had a significantly different mean total bottle volume in the vertical and horizontal positions, with all designs having greater volume in the vertical position.
- An adjusted ratio of mean number of drops/mean bottle volume demonstrated a range from 20.9 drops/ml to 40.8 drops/ml.
In summary, 20 drops per ml is a simplified figure for us to use in daily practice.
- Although it would be good if manufacturer provides the information on how many drops are there in each ml, most of them do not.
- The assumption of 20 drops per ml can lead to oversupply of glaucoma eye drops to patients in government health care facilities.
- Still, there will always be some patients who claim their eye drops run out earlier than expected. This could be due to a number of factors, such as elderly patients with trembling hands who may have difficulty administering the drops correctly, or patients who may be using more drops than they need.
Eye Ointments
Generally, eye ointments have a longer duration of effect than drops as drug dilution and drainage is slower.
- This makes them a good option for treating conditions that require long-lasting relief, such as dry eyes.
- Ointments also have the advantage of allowing the incorporation of drugs with poor aqueous solubility.
- However, it is more difficult to self-administer and often blurs the vision. Hence, in practice, eye ointments are more often to be given at night time.
Different from eye drops, we advise patients to squeeze a small amount of ointment or gel (about 1 cm length) into the pocket made by the lower lid, but do not let the tip of the tube touch eye, skin or lashes.
- Then, remove your index finger from the lower eyelid and blink your eye gently several times to spread the ointment; then close your eye for 1 to 2 minutes.
- Alternatively, after applying the eye ointment, patient may gently close the eye and roll the eyeball in all directions. Try not to blink and do not rub.
If patients need to use to eye drops and ointments at the same time of day, use the ointment last.
Eye Gels
Gels, which are semisolid system comprising water-soluble bases are also available and are more favourable than ointments for water-soluble drugs.
- They include polymers such as PVA, poloxamer, hydroxypropyl methylcellulose or carbomers dispersed in a liquid.
Gels that are activated by ions, pH and temperature have also been developed.
- These undergo a phase transition from liquid to solid in the conjunctival sac to form a viscoelastic gel.
- These in situ forming gels have an advantage over the preformed gels in that the dose is more reproducible and administration is easier, thus improving patient adherence.
Timolol maleate gel-forming solution (Timoptic-XE, Merck) contains a purified anionic hetero-polysaccharide derived from gellan gum.
- The gellan gum is in aqueous solution and forms a gel in the presence of cations which exist in the precorneal tear film.
- Timolol gel-forming solution is administered once daily, compared with twice daily for the regular timolol preparation to achieve a similar reduction in intraocular pressure.
Storage of Eye Drops
There are few commercial eye drops which have to be refrigerated at 2-8°C.
- Chloramphenicol 0.5% eye drops (Nicol, Shinacol, Xepanicol)
- Latanoprost 0.005% and timolol maleate 0.5% eye drops (Xalacom)
- Latanoprost 0.005% eye drops (Xalatan)
- Omidenepag Isopropyl 0.002% eye drops (Eybelis)
- Proparacaine HCl 0.5% eye drops (Alcaine)
- Tafluprost 0.0015% eye drops (Taflotan-S)
- Tafluprost 0.0015% and Timolol 0.5% eye drops (Tapcom-S)
For both "latanoprost 0.005% and timolol maleate 0.5% eye drops" and "latanoprost 0.005% eye drops", the usual recommended storage instruction is
- Prior to opening, store at 2 to 8°C.
- After opening, may store up to 25°C for 4 weeks.
- Protect from light.
The recommendation above is made based on a study which shows that latanoprost stored at 30°C for 4 weeks after opening the bottle remains as effective and safe as latanoprost stored under cold conditions.
On the other hand, a 2008 study concluded that inadequate refrigeration and prolonged shelf-lives of chloramphenicol generics collected from Delhi and Chennai are associated with very high levels of chloramphenicol thermal breakdown product.
- According to the same article, it highlights that chloramphenicol is thermo-labile, and it is recommended in the UK that chloramphenicol preparations are stored under refrigeration as per manufacturers’ instructions, to minimize thermal degradation.
- After 1 year’s storage in refrigeration (0-4°C), it has been demonstrated that there is nearly 20% less breakdown of chloramphenicol content in topical eye-drops to the hydrolysis product 2-amino-1-(4-nitrophenyl)propane-1,3-diol, when compared with drops stored for the same length of time at room temperature (20-25°C). The degree of thermal degradation would presumably be even greater at higher room temperatures, such as found in tropical countries.
Preservatives in Eye Drops
As an overview, preservatives are required to reduce contamination in most multidose eye drops.
- Some of the examples are benzalkonium chloride (BAK), polyquad (polyquaternium), sodium chlorite, Purite® (stabilized oxychloro complex), SofZia® and sodium perborate (GenAqua® and Dequest®).
- Benzalkonium chloride (BAK) is preferred by many manufacturers because of its stability, excellent antimicrobial activity and long shelf-life. Unfortunately, long-term use of topical products containing BAK can lead to damage of conjunctival and corneal epithelial cells. It is also not suitable for contact lenses.
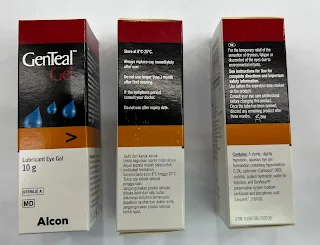
- Some products such as GenTeal are uniquely formulated to allow the preservative to rapidly dissociated into nontoxic components on the ocular surface.
Normally, tears quickly dilute and remove preservatives. However, if tear secretion decreases, the risk of preservative toxicity increases.
- Adverse effects include mild irritation (burning or stinging), symptoms of dry eye syndrome (with disruption of tear film), severe conjunctivitis and, rarely, corneal scarring.
- Toxicity is related to the type and concentration of preservative, the frequency and duration of use and whether the tear film is intact.
Preservative-Free Eye Drops
To maintain sterility without the use of a preservative, the design of eye drop bottles has been looked into by manufacturers.
Single use units are eye drops that come in individual containers that are only used once and are often preservative-free.
- Although single use units can be more expensive than multi-dose eye drops, they can be beneficial for people who are sensitive to preservatives.
ABAK® patented filter system uses a 0.2 μm polyether sulphone membrane with both hydrophilic and hydrophobic properties to prevent bacteria from entering the bottle.
The Airless Antibacterial Dispensing System (AADS™, Pfizer) works by preventing air, and therefore bacteria, from entering the container on dispensation.
- Furthermore, a silver coil is included in the bottle tip. Silver has antibacterial properties and therefore any bacteria contacting the tip do not contaminate the contents.
- This system guarantees 3 months of sterility in-use.
COMOD system (Continuous Mono Dose) developed by URSAPHARM is also an integrated airless application system.
- The contents remain sterile for a period of use of 6 months - all without preserving agents.
Aptar Pharma launched the Vismed Multi, featuring its patented preservative free Ophthalmic Squeeze Dispener.
- The system employs a purely mechanical approach to prevent microbial contamination of the product. It utilizes two key technologies: tip seal technology, a specially designed outlet valve, and sterile filtration of the venting air.
- After first use, both Vismed Multi and Vismed Gel Multi remain sterile for up to 3 months.
In the patented Pure Flow Technology by Alcon, the bottle has been designed with a one-way valve that ensures no contaminated liquid or air can be reintroduced into the container.
- This technology makes it possible to use a silicone membrane to filter the air and eliminates the need for preservatives in the eye drops.
- After use, before closing the cap on the bottle, shake the bottle downward to remove residual product that may be left on the tip.
- Can be used for up to 3 months after opening.
Expiry Date
For most commercial marketed eye products, the shelf life that we always tell our patients and customers is 28 days after opening, unless otherwise stated.
- However, a NPS article dated in 2008 challenge that this 28-day policy is based on research from earlier times when drops were dispensed in glass bottles with glass pipettes, and many eye drops did not contain preservatives. However, to author knowledge, none of this research is current, using modern dropper-type bottles. Plus, the policy seems a terrible waste and causes increased expense to the patients and the health system.
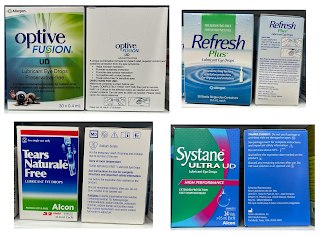
In year 2020, there is few interesting findings that I came to realize.
- The packaging of Systane Ultra now writes: discard any remaining solution SIX months after first opening. Earlier, the recommendation had been to discard one month after opening.
The in-use shelf life of GenTeal eye drop and eye gel is updated from 1 month to 3 months in 2023.
In 2023, I also came to realize that all single-use vials (preservative-free) are now recommended to discard immediately after each use, instead of 12 hours.
 |
| Old Packaging |
 |
| New Packaging |
NOTE: You can find more examples of in-use shelf life for different eye drops here.
Summary
Eye drops are challenging to administer and require coordination, manual dexterity and vision, necessitating clear instructions and patient counselling.
Patients with a three times daily regimen were more likely to miss doses and also had irregular timing of doses compared to patients with twice daily regimens.
- This illustrates the importance of designing ophthalmic preparations that provide a sustained drug release and which require less frequent dosing.
- A double-masked conjunctival allergen challenge (CAC) study in 2007 found no significant difference in the mean itching scores between two drops of olopatadine 0.1% (Patanol) and one drop of olopatadine 0.2% (Pataday). Both showed significant activity at the 24-hour time point and were statistically superior to placebo.
Moreover, there are many newer drops which can achieve same pressure reduction with better patient tolerability by using a lower concentration and improved delivery system.
- A 2014 study concluded that reductions in IOP from latanoprost baseline were larger with bimatoprost 0.01% than with bimatoprost 0.03%, and bimatoprost 0.01% had a more favourable tolerability profile.
- Brimonidine 0.15% and 0.1% drops (preserved with Purite®) appear to lower intraocular pressure to a similar extent as the 0.2% drops (preserved with benzalkonium chloride) and may cause fewer adverse effects.
- In a clinical trial, patients with open-angle glaucoma or ocular hypertension treated with IZBA (travoprost 0.03%) dosed once-daily in the evening, demonstrated intraocular pressure lowering equivalent to Travatan (travoprost 0.04%) eye drops at all on-therapy visits and time points (95% CI within +/-1.0 mm Hg) with a lower risk of hyperaemia.
External Links
- SafeMedication - How to use eye drops and eye ointment properly
- How to Use Eye Drops Properly, 2003
- On the correct use of eye drops, 2008
- Preserving Without Preservatives, 2006
- ABAK Pure Technology in a Bottle
- PRIMARY PACKAGING – Ophthalmic Squeeze Dispenser (OSD): Does One Size Fit All?, 2015
- The COMOD system. A preservative-free multidose container for eyedrops, 1994
- The COMOD® system – the safe solution for your eyes
- Alcon Readies Multi-Dose Bottle for Systane Complete PF, 2022











Comments
Post a Comment