Oral Administration
Introduction
Tablets are widely used and generally preferred by patients for various reasons, including
- Ease of swallowing.
- Portability and compactness (in comparison to liquid formulations).
- Unlike injectable dosage forms, they are non-invasive and can be self-administered.
The rate and extent of oral drug absorption are influenced by the
- Physiological factors associated with the structure and function of the gastrointestinal tract
- Physicochemical properties of the drug (e.g. solubility and dissolution rate) and dosage form factors
In general, for oral absorption, a molecule should not violate more than one of the rules known as Lipinski's Rule of Five. The molecule should have
- No more than 5 hydrogen bond donors (-NH, -OH)
- No more than 10 hydrogen bond acceptors (-N, -O)
- A molecular mass less than 500 Da
- An octanol-water partition coefficient, log P, of not more than 5
Exceptions to the rule of 5 are those probably substrates for naturally occurring transporters, such as antibiotics, antifungals, vitamins and cardiac glycosides.
Oral Formulations and Bioavailability
If a drug is administered intravenously, the bioavailability of the drug will be 100%.
- In comparison, the drug formulated for oral administration will undergo absorption and followed by first-pass metabolism.
- First-pass metabolism can also be avoided by drug administration via the mouth (buccal or sublingual) or via the rectum.
My colleague asked me a question that has been bugging her for a while. If a medication is available in oral tablet and syrup form, are they assumed to have same bioavailability?
For a drug which is administered orally to be 100% bioavailable, the entire dose must move from the dosage form to the systemic circulation. The drug must therefore
- Be completely released from the dosage form;
- Be fully dissolved in the gastrointestinal fluids;
- Be stable in solution in the gastrointestinal fluids;
- Pass through the gastrointestinal barrier into the mesenteric circulation without being metabolized; and
- Pass through the liver into the systemic circulation unchanged.
Many factors have been found to influence the rate and extent of absorption, and hence the time course of a drug in the plasma, and therefore at its site(s) of action. These include
- The foods eaten by the patient
- The effect of the disease state on drug absorption
- The age of the patient
- The site(s) of absorption of the administered drug
- The coadministration of other drugs
- The physical and chemical properties of the administered drug
- The type of dosage form
- The composition and method of manufacture of the dosage form
- The size of the dose
- The frequency of administration
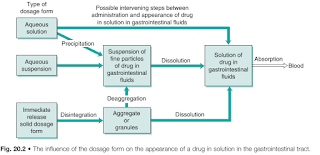
First and foremost, drugs must be in solution in the gastrointestinal fluids before absorption can occur.
- Thus the greater the number of intervening steps, the greater will be the number of potential obstacles to absorption and the greater will be the likelihood of that dosage form reducing the bioavailability exhibited by the drug.
- Hence, the bioavailability of a given drug tends to decrease in the following order: aqueous solution > aqueous suspensions > solid dosage forms (e.g. hard capsules or tablets). Although this ranking is not universal, it does provide a useful guideline.
- In general, solutions and suspensions are the most suitable for administration of drugs intended to be rapidly absorbed. However, it should be noted that other factors (e.g. stability, patient acceptability) can also influence the type of dosage form in which a drug is administered via the gastrointestinal route.
Back to practice, we often give oral tablet and suspension with the same dose interchangeably, however, we on the other hand should be more cautious when switching drug forms (especially those with narrow therapeutic windows).
- Amoxicillin and clavulanate
Oral preparations contain a mixture of amoxicillin and clavulanic acid, which the proportions of the two drugs in the medication could be different. Hence, the tablets cannot be directly converted to suspension. - Carbamazepine
Because a given dose of suspension will produce higher peak and lower trough levels than the same dose given as the tablet form, patients given the suspension should be started on lower doses given more frequently (same total daily dose) and increased slowly to avoid unwanted side effects. - Digoxin
Formulation dependent; Elixir: 70 to 85%; Tablet: 60 to 80%. - Emtricitabine
Capsule: 93%; solution: 75%. Relative bioavailability of solution to capsule: 80%. - Everolimus
Afinitor tablet bioavailability: ~30%. Systemic exposure reduced by 22% with a high-fat meal and by 32% with a light-fat meal.
Afinitor tablet for suspension bioavailability: AUC equivalent to tablets although peak concentrations are 20% to 36% lower; steady state concentrations are similar; Systemic exposure reduced by 12% with a high-fat meal and by 30% with a low-fat meal. - Itraconazole
Formulations are not interchangeable; generally, oral suspension is the preferred formulation because of greater absorption. Absorption of itraconazole from Sporanox capsules (and bioequivalent brands) requires acidic stomach pH (absorption from Lozanoc capsules and oral liquid is unaffected). - Mercaptopurine
Absorption is variable and incomplete (~50% of a dose is absorbed); Cmax of suspension is 34% higher than the tablet. - Posaconazole
Absorption of posaconazole from the oral liquid is more variable than from tablets and is more affected by the presence of food, e.g. when both are given on an empty stomach, bioavailability of the tablet is nearly 4 times greater than the oral liquid. - Raltegravir
600 mg film-coated tablet, chewable tablet and oral suspension have higher bioavailability compared to 400 mg film-coated tablet; dosage forms are not interchangeable. - Sodium fusidate
Fusidic acid is incompletely absorbed and doses recommended for suspension are proportionately higher than those for sodium fusidate tablets.
Pattern of Drug Release from Tablets
Most conventional (immediate) oral products (including both tablets and capsules) are formulated to release the active ingredient immediately after oral administration.
- This usually result in relatively rapid drug absorption and hence, onset of pharmacodynamic effects, but not always.
- For example, the pharmacodynamic activity of prodrugs may be altered by hepatic metabolism and lipophilic drugs absorption may gradual due to slow dissolution or selective absorption across the GI tract.
- Immediate release tablets include disintegrating, chewable, effervescent, sublingual and buccal tablets.
On the other hand, modified-release (MR) tablets should normally be swallowed intact and the pattern of drug release is deliberately changed. Types of MR drug products include, but not limited to
- Extended-release (ER) - allows at least a two-fold reduction in dosage frequency as compared to drugs presented as immediate-release (conventional) dosage form.
- Delayed-release (e.g. enteric coated) - releases a discrete portion/portions of drug at a time other than the promptly release after administration.
- Targeted-release - releases drugs at or near the intended physiologic site of action.
- Repeat-action - release one dose of drug initially, followed by a second or more doses of drug at a later time.
# Terms, such as ER (extended-release), SR (sustained-release), XL (another abbreviation for extended release), XR (extended-release) and CR (controlled release) are used to indicate the mechanism of the extended-release drug product employed. Retarded release is an older term for a slow-release drug product.
Tablet Types
Conventional or plain tablet is the most common type of tablet and is intended to be swallowed and to release the drug a relatively short time thereafter by disintegration and dissolution.
Chewable tablets are chewed and thus are mechanically disintegrated in the mouth. The drug is, however, normally not dissolved in the mouth but swallowed and dissolves in the stomach or intestine. Thus, chewable tablets are used primarily to accomplish a quick and complete disintegration of the tablet - and hence obtain a rapid drug effect - or to facilitate the administration of the tablet. A common example is antacid tablets. Another general advantage of a chewable tablet is that this type of medication can be taken when water is not available. Due to palatability concern, sweetening and flavouring agents are commonly included.
Effervescent tablets are dropped into a glass of water before administration, during which carbon dioxide is liberated. This facilitates tablet disintegration and drug dissolution; the dissolution of the tablet should be complete within a few minutes. Effervescent tablets are used to obtain rapid drug action or to facilitate the intake of the drug.
Lozenges (also known as pastilles or troche) are tablets that dissolve slowly in the mouth and so release the drug dissolved in the saliva. Lozenges are used for systemic drug uptake or for local medication in the mouth or throat, e.g. with local anaesthetic and antiseptic. Disintegrants are not used in the formulation.
Orally disintegrating tablets (ODTs) are capable of turning quickly into a liquid dosage form when in contact with the saliva, thus possessing the advantages of both the solid dosage forms particularly stability and liquid dosage forms especially ease of swallowing and pregastric absorption of drug.
Sublingual tablets and buccal tablets are used for drug release in the mouth followed by systemic uptake of the drug. A rapid systemic drug effect can thus be obtained without liver first-pass metabolism. Sublingual tablets are placed under the tongue and buccal tablets are placed in the side of the cheek or high up between the inside of the upper lip and gum.
Capsules
Capsules are soluble shells of gelatin (pork-derived) or hypromellose (a vegetable product), which are filled with the active drug, diluents (fillers) and any other excipients.
Capsules are tasteless and odourless, allowing them to be used to mask the unpleasant tastes and/or odours of some drugs, and for some patients, they are easier to swallow compared with tablets.
Salt Forms
Dissolution rate of a weakly acidic drug in gastric fluid (pH 1-3.5) is low, because mainly unionized. Hence, oral administration of a solid dosage form with a strong basic salt of a weakly acidic drug increase dissolution and therefore increase absorption compared with free acid.
- Since naproxen is a weak acid (pKa = 4.15), the sodium salt of naproxen was developed since it will provide more rapid absorption of naproxen and hence, an earlier effect.
- Ibuprofen is another classic example (i.e. same therapeutic effect, but different absorption rate).
- Diclofenac sodium and diclofenac potassium - The main difference between the two is that diclofenac potassium is absorbed into the body more quickly than diclofenac sodium. A quick action is useful where immediate pain relief is required, and a prolonged action is more useful in reducing inflammation.
Due to differences in absorption, 400 mg erythromycin ethyl succinate produces the same serum levels as 250 mg erythromycin base or stearate.
Perindopril is marketed in two different salt forms where 2.5 mg of perindopril arginine is equivalent to 2 mg of perindopril erbumine. Is there any difference in bioequivalences between perindopril erbumine and arginine?
- The short answer is no difference. They are interchangeable after dose normalization.
- However, perindopril arginine (the newer salt form) has improved stability at higher temperature or humidity.
- In government facilities, we only keep the perindopril erbumine form.
Method of Administration and Onset
An interesting study is carried out to investigate the best method of aspirin administration for heart attack aid. To find out how aspirin works fastest, researchers in Texas asked 12 volunteers to take a standard 325-mg dose of aspirin in three different ways:
- by swallowing a tablet with 4 ounces of water,
- by chewing the tablet for 30 seconds before swallowing it, or
- by drinking 4 ounces of water with Alka-Seltzer.
Each subject tried all three methods on an empty stomach on different days. The scientists monitored blood levels of aspirin and its active ingredient, salicylate, at frequent intervals, and they also measured thromboxane B2 (TxB2), an indicator of platelet activation that drops as platelets are inhibited.
By all three measurements, chewed aspirin worked fastest. It needed only 5 minutes to reduce TxB2 concentrations by 50%; the Alka-Seltzer took almost 8 minutes, and the swallowed tablet took 12 minutes. Similarly, it took 14 minutes for the chewed tablet to produce maximal platelet inhibition; it took Alka-Seltzer 16 minutes and the swallowed tablet 26 minutes.
Split Medication?
A separate blog post is published to discuss on issue of can an oral medication be split.





Can Wincardia (Aspirin) be placed under the tongue and dissolved with water since it is a dispersible tablet?
ReplyDeleteThere is a distinction between dispersible tablet and orodispersibe tablet or sublingual tablet. Given Wincardia is a dispersible tablet, it can be either dispersed in water immediately before use or swallowed whole.
Delete