Poisons Act 1952 and Regulations
Introduction
All things are poison, and nothing is without poison, the dosage alone makes it so a thing is not a poison. -- Paracelsus
On 21 July 2022, The Dewan Rakyat approved amendments to the Poisons Act 1952 that raises penalties and enhances enforcement powers.
- Poisons (Amendment) Act 2022 is officially commenced on 1 January 2023, except section 21 (which amends section 32 of Poisons Act 1952 on compounding of offences).
Today, we are going to look at few practical aspects of Poisons Act 1952 and Regulations:
- Community pharmacy
- Type A license
- Sales of Poisons by Retail
- Prohibition of Sale to Persons Under 18
- Poison Groups
- Group B Poisons
- Group C Poisons
- Exempt
- Prescription
- Prescription Book (Group B and Group C Poisons)
- Recording of Tablet or Capsule Formulations Containing Codeine, Dextromethorphan, Ephedrine and Pseudoephedrine
- Hospital pharmacy
- Powers of Minister of Health
Type A License
As stated in Section 26 of Poisons Act 1952, a Type A license is issued to a pharmacist to import, store and deal generally by wholesale and retail OR by wholesale only OR by retail only.
- Also, according to Section 15(2)g of Poisons Act 1952, sales of poison should only be sold by a licensed wholesaler to a licensed pharmacist at the business premise.
Type A License needs to be renewed annually.
- Starting 1st January 2019, License A fees will be RM 300 per license.
Sales of Poisons by Retail
As stated in Section 16 of Poisons Act 1952, a poison can be sold by retail by a licensed pharmacist or a registered pharmacist employed in the premise.
- According to Section 16(4A), every licensed pharmacist shall keep records of a registered pharmacist engaged or employed in a premises where the licensed pharmacist is licensed to retail poisons in accordance with any regulations made under Poisons Act 1952,
In other words, locum pharmacists can practice at community pharmacy without having to apply license A, as long as that pharmacy has a licensed pharmacist.
- Subsequently, a registered pharmacist may not hold more than one License A for different premises simultaneously.
NOTE: Prior to Poisons (Amendment) Act 2022, only licensed pharmacist can dispense Group B poisons. Hence, locum pharmacists have to apply license A to dispense legally.
Prohibition of Sale to Persons Under 18
As stated in Section 17 of Poisons Act 1952, no poison shall be sold or supplied to any person under 18 years old, otherwise than for purposes of the medical treatment of such person.
Poison Groups
The legal classification used in a country will control how medicines and poisons are made available to the general public. At Malaysia, supply of poisons is subdivided into 4 groups in accordance to Poisons Act 1952.
- Group A - by a licensed wholesaler to another licensed pharmacist/wholesaler or to a purchaser outside Malaysia
- Group B - by registered medical practitioner/registered dentist Division I/registered veterinary surgeon OR by a registered pharmacist as a dispensed medicine in accordance with a prescription from a licensed pharmacy
- Group C - as a dispensed medicine
- Group D
The complete list can be found in the First Schedule of the Poison List.
- Alternatively, you may also find the information under "regulatory classification" section in MIMS Malaysia.
NOTE: As stated in Section 6 of the Poisons Act 1952, Minister of Health may, from time to time, after consultation with the Poisons Board to amend the Poisons List.
Group B Poisons
As stated in Section 21 of the Poisons Act 1952, a registered pharmacist is authorized to sell Group B poisons by retail to any person in accordance with a prescription prescribed by a registered medical practitioner, registered dentist or registered veterinary surgeon.
Group C Poisons
On the other hand, Group C poisons can be supplied by a community pharmacist to patients without prescription.
- Group C poisons can still be prescribed by a registered medical practitioner/registered dentist Division I/registered veterinary surgeon on a prescription.
Below are examples of Group C poisons that you may encounter in community pharmacy setting.
- Acarbose
- Topical acyclovir containing not more than 5% w/w
- Colchicine except for veterinary preparations
- All nicotine preparations except tobacco
- Aminophylline
- Amiodarone; its salts
- Amorolfine preparations for external use containing more than 5% w/w
- Antibiotic suppositories and topical preparations for nose, eyes and ears. Lozenges and preparations for external use only.
- Antihistamines, e.g. azelastine, bilastine, carbinoxamine, cetirizine, chlorpheniramine, cinnarizine, desloratadine, dimenhydrinate, diphenhydramine, ebastine, emedastine, fexofenadine, levocetirizine, loratadine, meclozine, olopatadine, promethazine, triprolidine
- Azelaic acid preparations for external use
- Benzydamine
- Betahistine
- Bifonazole preparations for external use
- Calcipotriol
- Calcitriol unless exempted
- Chloramphenicol topical preparations for nose, eyes and ears.
- Cimetidine
- Cloperastine
- Clotrimazole pessaries
- Cortisone, hydrocortisone and their derivatives/analogues topical preparations for nose, eyes, ears, mouth or throat
- Cyclopentolate
- Deferiprone
- Desferrioxamine
- Dextromethorphan
- Diclofenac
- (DD) Diphenoxylate registered under the Control of Drugs and Cosmetics Regulations 1984
- Dithranol
- Econazole pessaries and preparations for external use
- Erdosteine
- Ethambutol
- Ethionamide
- Famotidine
- Fenoterol except for use in animal feeds
- Fluconazole pessaries and preparations for external use
- Formoterol except for use in animal feeds
- Glibenclamide
- Gliclazide
- Glimepiride
- Glipizide
- Glyceryl trinitrate
- Glycopyrrolate
- Griseofulvin
- Hydroxychloroquine
- Hydroxyzine
- Hyoscine
- Ibuprofen
- Imiquimod for external use
- Insulin
- Iodine preparation for parenteral administration or medicinal preparations containing 2% and above
- Ipratropium
- Isoconazole pessaries and preparations for external use
- Isoniazid
- Isosorbide dinitrate
- Isosorbide mononitrate
- Itraconazole pessaries and preparations for external use
- Ketoconazole pessaries and preparations for external use
- Ketotifen
- Local anaesthetics for topical use in the nose, eyes and ears or external use, e.g. benzocaine, cinchocaine, lignocaine, prilocaine.
- Loperamide
- Mebeverine
- Mefenamic acid
- Melatonin
- Meloxicam
- Metformin
- Miconazole pessaries and preparations for external use
- Minoxidil preparations for external use, not more than 5%
- Montelukast
- Naproxen
- Nepafenac
- Nystatin
- Orlistat
- Oxybutynin
- Oxymetazoline
- Paracetamol for parenteral administration
- Penciclovir topical use
- (DD) Pholcodine registered under the Control of Drugs and Cosmetics Regulations 1984
- Pimecrolimus
- Piroxicam
- Pizotifen
- Pyridostigmine
- Racecadotril
- Ranitidine
- Repaglinide
- Rosiglitazone
- Salbutamol except for use in animal feeds
- Salmeterol except for use in animal feeds
- Selenium sulphide unless exempted
- Sertaconazole preparations for external use
- Sodium cromoglycate
- Solifenacin
- Tacrolimus preparations for external use
- Telbivudine
- Terbutaline except for use in animal feeds
- Theophylline medicinal preparations
- Tibolone
- Tiotropium bromide
- Tolbutamide
- Tolterodine
- Tretinoin
- Trimetazidine
- Tropicamide
- Trospium chloride
- Ulipristal
- Urea in medicinal preparation containing 40% and above
- Ursodeoxycholic acid
- Varenicline tartrate
- Verapamil
- Vilanterol
- Xylometazoline
NOTE: Few antidiabetic and inhaler medications are listed as Group C Poisons.
Exempt
If you look carefully at the First Schedule of the Poison List, there are certain poisons fall under Exempt, in which we can sell them over the counter in community pharmacy or elsewhere:
- Acetylcysteine except for parenteral administration
- Amorolfine preparation for external use and not more than 5%
- Amino acids except for parenteral administration
- Bromhexine except for parenteral administration
- Calcitriol product with is registered under the Control of Drugs and Cosmetics Regulations 1984 except for parenteral administration
- Cholecalciferol except for parenteral administration or containing 10000 IU or more per dosage unit
- Citicoline except for parenteral administration
- Clotrimazole preparation as single ingredient for external use
- Electrolytes except for parenteral administration
- Glucose except for parenteral administration
- Glutathione except for parenteral administration
- Iodine except for parenteral administration or containing 2% and above
- Minerals except for parenteral administration, e.g. chromium, copper, fluorine, iron, manganese, selenium, zinc
- Nicotine in tobacco
- Paracetamol except for parenteral administration
- Potassium permanganate preparations containing 0.1% and less
- Selenium sulphide containing 1% w/v or less for external use
- Terbinafine preparation for external use
- Triglycerides except for parenteral administration
- Valerian root
- Vitamins except for parenteral administration
Prescription
As stated in Section 24 of Poisons Act 1952, prescription could refer to oral or written instruction by registered prescriber.
- If any prescription is given orally, such prescription shall be confirmed by a written prescription within 1 day.
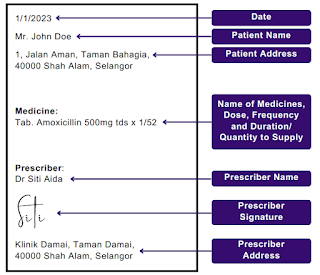
As stated in Section 21 of Poisons Act 1952, the minimum requirement of every written prescription for any Group B Poison shall:
- Be in writing, signed and dated by the prescriber thereof;
- State the name and address of the prescriber;
- State the name and address of the patient or, in the case of a prescription by a veterinary surgeon, the name and address of the person to whom such medicine is to be delivered;
- Indicate the total amount of medicine to be supplied and the dose;
- Specify the number of times (not exceeding 3) the medicine may be dispensed and, if dispensed more than once, at what intervals; and
- Not be written wholly or partly in code or in such manner that it is not readily decipherable and capable of being dispensed by any pharmacist
NOTE: No person shall sell or supply by retail any Group B Poison on a prescription which does not comply with all the requirements above.
As stated in Section 21(2A) and (2B), electronic prescription for any Group B poisons prescribed shall
- Be created and date in electronic form;
- Be signed with a digital signature by the prescriber (in accordance with the Digital Signature Act 1997);
- Be sent to a registered pharmacist as an electronic message;
- Fulfils all the minimum requirements for a manual prescription.
NOTE: Only till commencement of Poisons (Amendment) Act 2022, electronic prescription is considered a legal prescription.
At the time of selling or supplying any Group B poison, every registered pharmacist shall endorse or mark the prescription in a manner so as to permanently attach to the prescription, his name and address and the date on which such poison was sold or supplied.
Emergency Supply
As Stated in Section 21(6) of Poisons Act 1952, for emergency cases, it shall be lawful for the seller, after making an entry to that effect in his Prescription Book, upon the verbal or telephoned instructions of a registered medical practitioner, personally known to him, to supply poison without such prescription.
- Provided that in every such case the seller or supplier shall take all necessary steps to obtain, and the prescriber shall deliver a written prescription within 1 day of the date of such sale or supply.
Prescription Book (Group B and Group C Poisons)
Section 24 of the Poisons Act 1952 states that on the day on which such poison is sold as a dispensed medicine, the transaction should be entered in a register (referred to as the "Prescription Book").
- The date on which the medicine was sold or supplied and the serial number of the entry in such register of the prescription (if any);
- The name of the poison and the ingredients of the medicine or, in the case of a proprietary medicine, the name of the medicine and the quantity supplied;
- In the case of a sale or supply by a retailer on a prescription, the name of the patient, or, when the prescriber is a registered veterinary surgeon, or the prescription relates to animal treatment, the name of the recipient.
Provided that when a prescription is repeated, it shall be sufficient to enter in the Prescription Book
- The date
- The serial number of the sale, supply and prescription (if any) originally entered and
- The name of the patient or recipient.
NOTE: It is a common practice to use a separate Prescription Book for Group B poisons and Group C poisons.
Recording of Tablet or Capsule Formulations Containing Codeine, Dextromethorphan, Ephedrine and Pseudoephedrine
Subject to Section 26(4) of Poisons Act 1952, all transactions involving tablet or capsule formulations containing codeine, dextromethorphan, ephedrine or pseudoephedrine should also be recorded.
- Recordings are made on the day of transaction itself.
- Any corrections should be made with a side or bottom note (with the correction date).
- The transactions should be recorded in chronology order.
- The recording book is kept at licensed premise.
NOTE: The transaction includes stock receiving as well as supplying of the poison, and the transaction should be recorded on different pages for each item. It is also a common practice to "check and found" for such item groups regularly to ensure the quantity recorded and the actual quantity on hand are the same.
Hospital Pharmacy
Interestingly, under the section 7(3) of Poisons Act 1952, it mentions that this Act shall not apply to the sale or supply of any poison or of any medicine containing poison by any officer or person, who
- Is employed in any hospital, infirmary, dispensary or veterinary hospital wholly maintained by the Government of Malaysia or any State Government, or by any local authority, or out of public finds or by a charity approved by an order of the Director General of Health, and who sells or supplies in the course of his duty such poison or medicine to any outpatient of such hospital, infirmary or dispensary for the medical or dental treatment of such patient or, in the case of an officer or person employed in a veterinary hospital, to any person for the animal treatment of any animal tended by him; or
- Is employed in any hospital, infirmary, dispensary, clinic, nursing home or other institutions at which human ailments are treated, and who sells or supplies in the course of his duty such poison or medicine for the use in the wards, operating theatres or other sections thereof;
Provided that such sale or supply is made and conducted in accordance with any regulations expressly applicable thereto made under this Act.
According to Regulation 23 of Poisons Regulation 1952, the poison shall only be supplied to out-patients in hospitals by, or on and in accordance with a prescription. In case where a poison is supplied, a record shall be kept on the premises in such a way that there can readily be traced at any time during a period of 2 years after the date on which the poison was supplied with the following particulars:
- The name and quantity of the poison supplied.
- The date on which the poison was supplied.
- The name and address of the person to whom the poison was supplied
- The name of the person who supplied the poison or who gave the prescription upon which it was supplied.
Powers of Minister of Health
Section 3 Appoint 12 members of the Poisons Board.
Section 6 Amend Poisons List from time to time, after consultation with the Poisons Board.
Section 24(4) & 5 Make decision of appeal related to license issued.
Section 32A With the approval of the Public Prosecutor, make regulations prescribing any offence under Poisons Act 1952, and the method and procedure for compounding such offence.
Section 35 Make regulations to carry out the purposes of Poisons Act 1952.
Disclaimers
Poisons Board, licensing officer and drug enforcement officer under Poisons Act 1952 is discussed separately.
Anything found in the third schedule of the Poisons Act 1952 will be regulated under Poisons (Psychotropic Substances) Regulations 1989, which is discussed separately.
Dangerous Drugs Act 1952 and Regulations is discussed separately.
External Links
- Poisons Act 1952 and Regulations
- SOALAN LAZIM RANG UNDANG-UNDANG RACUN (PINDAAN) 2022
- Amendment of Acts enforced by Pharmaceutical Services Division, Ministry of Health Malaysia
- Revise Flawed Poisons Act (Amendment) Bill 2022, 21 March 2022
- Doctors fear abuse of proposed new powers for pharmacy officers, 28 March 2022
- In Poisons Bill Debate, MPs Question Enforcement Powers, Harsh Penalties, 21 July 2022
- Parliament Passes Poisons Amendment Bill After Three-Year Delay, 21 July 2022
- FAQs Related to Licensing Activities with the Enforcement of the Poisons (Amendment) Act 2022, 2023
Hello. Does the prescrpition book a requirement when dispensing Group B and Group C poison? or is it just for group B only?
ReplyDeleteIf we look at Poison Act 1952, the section 24 mentions that prescription book is a requirement for any poisons that is sold under dispensed medicine. Hence, it will be a requirement for both poison B and poison C. This is what being practised in community pharmacy too.
DeleteSection 22(b) of poison act 1952 clearly mentioned that provision of poison c by a pharmacist is considered as a dispensed medicine too. =)
DeleteHi, can you explain what are the differences between licensed pharmacist and registered pharmacist?
ReplyDeleteA registered pharmacist is a pharmacist that already registered within Pharmacy Board as per Registration of Pharmacist Act 1952.
DeleteA licensed pharmacist is a registered pharmacist who have applied and successfully obtained licence A for the given premise (under Section 26 of Poison Act 1952).
In other words, you can think as for any registered pharmacist to be lawfully working in a community pharmacy, he first needs to apply licence A for the intended premise. Only after approval of the licence A, he is a licensed pharmacist for that particular community pharmacy.
With commencement of Poisons (Amendment) Act 2022 in 1 January 2023, a registered pharmacist can practice at community pharmacy without having to apply license A, as long as that pharmacy has a licensed pharmacist.
DeleteHi, can you explain the differences between first , second , third and fourth schedule poison?
ReplyDeleteAs an overview, there are 3 schedules under Poisons Act 1952.
DeleteFirst schedule is the schedule that we often refer to. It includes the list of Group A, Group B and Group C poisons, as well as exempted product.
Second schedule is substances where Poisons Act 1952 is not applicable, as stated in Section 7.
Third schedule is the list of psychotropic drugs where Psychotropic Substances Regulation 1989 applies.
Okayy thank youuuu for the information!
DeleteHi, is there any restriction on a company purchasing drugs in bulk?
ReplyDeleteFor company acting as wholesalers, they would have to apply licence A also. The requirements in psychotropic drugs applies too.
Deletehi there, I wanted to clarify about Group A poisons. Can Group A poisons be supplied by retail from pharmacists as a dispensed medicine for medical treatment of patients?
ReplyDeleteAs highlighted in Poisons Act 1952, it is not allowed.
Deletehi may i know what licenses (other than License A) and basic legal requirement are needed to operate a retail pharmacy?
ReplyDeleteBased on Panduan Permohonan Lesen Jenis A di bawah Akta Racun 1952, other requirements may include registration with Suruhanjaya Syarikat Malaysia (SSM) as required by Registration of Businesses Act 1956, registration of "Body Corporate" as required by Registration of Pharmacists Act 1951 and "Lesen perniagaan" from "Pihak Berkuasa Tempatan" (PBT).
DeleteHi, may i know what's the difference between poison book and prescription book? Thanks!
ReplyDeleteWhen a drug is sold as Group B or Group C poison (i.e. as a dispensed medicine), it needs to be recorded in the Prescription Book. On the other hand, when a drug is sold as Group D poison (e.g. for laboratory use), it needs to be recorded in the Poisons Book.
DeleteHi may i know the differences of Type A, B, E and permit? I dont really understand the description stated in the book...
ReplyDeleteTo simplify it, licensing officer is the person responsible to issue different licenses, which include
Delete- Type A - for pharmacists to import, store and sell poisons by wholesale or retail
- Type B - for wholesalers (not a pharmacist) to import, store and sell poisons
- Type D - for retailers to store and sell Part II poisons
- Type E - Dealing with sodium hydroxide
To identify if a poison is Part I or Part II poisons, you have to refer the first schedule of the Poisons Act 1952.
- Part I covers Poisons Group A, B, C and D.
The information is discussed separately at another article.
Deletei want to ask, is a pharmacist want to open a pharmacy, which type of license that he/she need to apply?
ReplyDeleteThis question is well addressed in another article.
DeleteIf the poison to be sold to manufacturer and non lab purpose, does it need to be recorded in poison book?
ReplyDeleteTo be honest, I am not familiar with it, but probably yes.
Deleteok
ReplyDeleteThe Poisons Act 1952 and its regulations in Malaysia govern the handling, sale, and distribution of substances classified as poisons. These regulations are critical for ensuring public safety and proper management within the healthcare and pharmaceutical sectors. Adhering to these guidelines is essential for maintaining standards of health and safety across the country. https://australianconceptkarachi.com/
ReplyDeleteSebuah syarikat ingin mengimport NaOH 10%. Apakah lesen yang perlu diberikan kepada syarikat tersebut?
ReplyDeletesince its fall under 12% (exempted in part ii posion) do they need license?
DeleteIn accordance with Poisons (Sodium Hydroxide) Regulation 1962 and its Regulation 2, a permit to purchase, store and use of sodium hydroxide is only required for any substance containing not less than 12% sodium hydroxide. However, Type A, Type B or Type E license is still required.
Delete