Labelling of Dispensed Medicine
Introduction
Guide to Good Dispensing Practice, 2016 is developed to ensure that medicines are dispensed in accordance with the laws and guidelines in both government and private health care facilities in Malaysia.
Labelling Requirements
All dispensed medicine should be labelled according to the requirement stated by the law.
- Regulation 12 of Poisons Regulations 1952
- Regulation 28 of Poisons (Psychotropic Substances) Regulations 1989
Label should contain
- Name, address and contact number of hospital/clinic/pharmacy
- Patient's name
- Name of medicines (generic and/or trade names)
- Dosage form with the strength and quantity per unit dosage form: mg/ml of liquid, mg/g for semi-solid preparations
- Directions for use: dose, frequency and duration (if necessary)
- Date of supply
- Expiry (especially if dispensed medicine is not in its original packaging)
- "Controlled Medicine" or "Ubat Terkawal" should be labelled for all controlled medicines
- Medicines for external use should be dispensed in suitable containers and should be labelled conspicuously with the words "Not to be Taken" or "For External Use Only" in Bahasa Malaysia and/or English printed in red OR on a red background
- Special precautionary labels should be used where necessary (e.g., "Complete the course" for antibiotics, "May cause drowsiness" for sedating drugs, etc.)
NOTE: It is advisable for labels to be printed. If handwritten, it should be neat and legible with clear instructions on use.
Patient-Centred Labels
Many studies have reported a high prevalence of patient misunderstanding of common prescription drug label instructions, and associated with unintentional medication misuse and adverse health outcomes.
- Consequently, a patient-centred label strategy is introduced to promote appropriate medication use and adherence.
Suggestions include
- Use larger font sizes (e.g. 12 point and above).
- Present complex information in lists rather than paragraphs when possible.
- Simplify language, avoiding unfamiliar words or medical jargon.
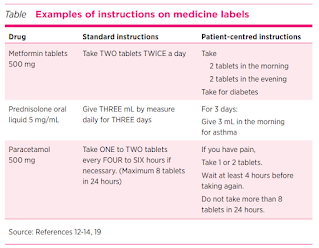
- Use numbers rather words to convey numeric information, for example 'take 2 tablets...' rather than 'take TWO tablets...'.
- Provide explicit dosing instructions, for example 'take 2 tablets in the morning, and take 2 tablets in the evening' rather than 'take TWO tablets TWICE a day'.
- Use white space and typographical cues (e.g. capitals, bolding and highlighting) to communicate important information.
- Use standard dosing times for medicine administration, for example 'morning, noon, evening, bedtime' rather than 'FOUR times daily' or 'every SIX hours'.
- Include the indication for the medicine when possible, for example 'if you have pain', rather than 'if necessary'.
- Include the maximum dose, if relevant, such as for paracetamol.
NOTE: The dispensing label must not obscure the active ingredient(s), strength, and expiry date as printed on the manufacturer's packaging.
Visually Impaired-Friendly Medicine Label Initiative
In 2023, Malaysia Ministry of Health has introduced the Visually Impaired-Friendly Medicine Label Initiative (as an extension of the Know Your Medicines Programme) to help visually impaired patients in taking medicines.
- This initiative would be implemented in 250 public health clinics nationwide that will benefit 55000 visually impaired persons.
- Through this initiative , drug labels given to visually impaired patients will have Braille writing to explain method of taking (i.e. drug dosage) and the indications of medication (e.g. fever, cough or cold).
Use Transparent Flag Labels for Smaller Containers
For small containers (such as eye drops), use a transparent flag label adhered to a full-size standard dispensing label. Using a flag label:
- Avoids the need to cut or fold the label
- Ensures that all label information on both the dispensed label and the manufacturer’s label remains visible.
- Allows the dispensed label to be applied directly to, and remain with, the manufacturer’s primary pack.
Labels that are folded in half and attached to a small container are difficult to read and can easily be torn off the medicine container.
- Using a transparent flag label keeps the label flat and maintains readability for consumers.
NOTE: While not yet widely implemented within the Ministry of Health facilities, the transparent flag label strategy presents a potential improvement for medication safety.
Summary
The implementation of PhIS system at pharmacies enables a standardized and consistent order of elements within pharmacy dispensing label across various Ministry of Health facilities.
- This consistent display format and order enhances consumer readability, minimizing the potential for confusion and misinterpretation.
- In addition to explicit and clear dosing instructions and drug information, auxiliary warning statements should also be included.
External Links
- Guide to Good Dispensing Practice, 2016
- Expanding the Universal Medication Schedule: a patient-centred approach, 2014
- A Patient-Centered Prescription Drug Label to Promote Appropriate Medication Use and Adherence, 2016
- Safer dispensing labels for prescription medicines, 2018
- National Standard for Labelling Dispensed Medicines, 2021
- MOH introduces visually impaired-friendly medicine labels, 2023


Common Medications Study Guide Bundle with Pharmacology Flashcards and Stickers | Medication Administration | Pharmacology Revision
ReplyDeletepharmacology stickers
hi
ReplyDelete