TDM for Specific Drugs
Introduction
Please refer Clinical Pharmacokinetics Pharmacy Handbook for complete information of individuals drug on
- Pharmacokinetics
- Dosage
- Interaction
- Sampling
- Monitoring parameter
- Adverse drug reaction
- Dilution and administration
- Calculation
Aminoglycosides
Aminoglycosides include amikacin and gentamicin.
- Is hydrophilic drug.
- If patient if fluid overload, it is important to correct the fluid balance and resample.
- Exhibit concentration-dependent bactericidal activity.
- Post level relates efficacy of the drug.
- Pre-level, on the other hand, show how well the drug is cleared from the body.
- The clearance of aminoglycosides is directly related to renal function.
Monitoring parameter, such as temperature, white blood cell count, C-reactive protein and culture & sensitivity result.
- Indicates clinical response of the patient.
Toxic effect: Nephrotoxicity and ototoxicity.
- Ototoxicity is more likely when co-prescribed with loop diuretics (e.g. frusemide) or vancomycin.
- Nephrotoxicity is more likely when co-prescribed with cyclosporin, platinum chemotherapy, cephalosporins or vancomycin.
Carbamazepine
Indications
- Treatment of partial and secondary generalized tonic-clonic seizures, primary generalized tonic-clonic seizures
- Trigeminal neuralgia, and
- Prophylaxis of bipolar disorder unresponsive to lithium
Autoinduction
- Carbamazepine induced its own metabolism via the hepatic microsomal enzyme CYP3A4 system.
- Onset is as early as 24 hours, and the time to completion has been reported to range from 1 to 5 weeks.
- Thus, for initial dose, patient is started on ¼ - ⅓ of the desired maintenance dose to avoid side effects of early therapy and taper up similar amount every 2-3 weeks until the total desired daily dose.
- The time to steady state depends on the completion of auto-induction.
Side effects
- Dose-independent, e.g. aplastic anaemia and Stevens-Johnson syndrome
- Individuals of Han Chinese or Thai origin should be screened for the HLA-B*1502 allele before starting carbamazepine treatment because it increases the risk of developing severe Steven-Johnson syndrome.
- Dose-dependent
- > 8 mg/L - CNS adverse effects increase (e.g. drowsiness, dizziness and headaches)
- 11-15 mg/L - somnolence, nystagmus and ataxia
- 15-25 mg/L - combativeness, hallucinations, chorea
- >25 mg/L - seizures and coma
Carbamazepine is known to cause blood dyscrasias including aplastic anaemia, agranulocytosis, leukopenia, thrombocytopenia, anaemias, and pancytopenia.
- Monitor complete blood count before initiating therapy and periodically thereafter.
Digoxin
Therapeutic range
- Chronic Heart Failure: 0.5-0.9 mcg/L
- Positive inotropic effects of digoxin were seen with low digoxin concentration hence the lower therapeutic range is used in CHF. This lower target range is based on the fact that most patients with CHF do not demonstrate additional therapeutic benefits from higher digoxin concentration.
- Atrial Fibrillation: 0.8-2.0 mcg/L
- Since the goal for digoxin in AF is rate control, hence higher therapeutic concentration is needed. A serum concentration of >1.2 mcg/L may be associated with increased all-cause mortality in patients with atrial fibrillation (regardless of heart failure). Each increment of 0.5 mcg/L in serum digoxin concentration from the baseline concentration was associated with higher risk of death.
- Digoxin maximum target concentration of 2 mcg/L was determined based on toxicity rather than efficacy. Heart rate at all levels of exercise in most patients with chronic AF is not adequately controlled by any therapeutic concentration of digoxin for which combination with other rate control agents should be considered.
There are a variety of important drug-drug interactions with digoxin. Some are relatively easily avoided, while others are not.
Patients with acute poisoning may develop severe bradycardia, heart block, vomiting, and shock.
- Hyperkalaemia is a marker of severe acute toxicity.
- Digoxin-specific Fab is the definitive therapy for digoxin toxicity and is indicated with life-threatening or potentially life-threatening toxicity.
- It must be noted that serum digoxin concentrations are no longer useful after DSFab administration unless free digoxin concentrations can be measured. The standard digoxin assay measures both free and bound digoxin and will measure the DSFab-digoxin complex in addition to both bound and unbound digoxin, which will lead to a falsely elevated value that will not likely correspond with clinical status.
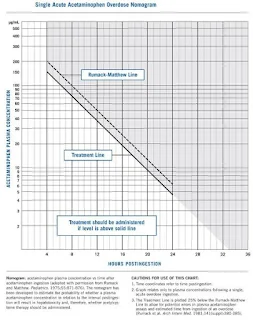
Paracetamol
Once an overdose occurs (whether intentional or unintentional), there could be a risk of paracetamol poisoning (which can lead to hepatotoxicity).
- Blood sample should only be drawn at least 4 hours after the acute ingestion.
- Also monitor aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels.
If the results fall within toxic range in Rumack-Matthew Nomogram, N-acetylcysteine (antidote) will be prescribed.
- The nomogram cannot be used if the patient presents more than 24 hours after ingestion or has a history of multiple paracetamol ingestions. Its reliability also decreases for ingestions of extended-release paracetamol formulations.
Phenytoin
The main route of elimination is via hepatic metabolism. However, this metabolic route can be saturated at normal therapeutic doses.
- This results in the characteristic non-linear dose-concentration curve.
- Therefore, instead of the usual first-order pharmacokinetic model, a Michaelis-Menten model, used to describe enzyme activity, is more appropriate.
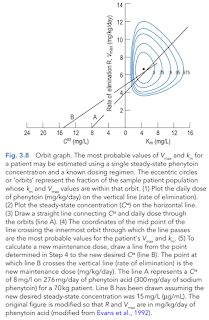
Alternatively, a nomogram (orbit diagram) may be used to assist in dose adjustments.
Care is needed when interpreting TDM data when
- Phenytoin is given concurrently with other anticonvulsants
- These affect distribution and metabolism of phenytoin, e.g. valproic acid may displace phenytoin, resulting more free phenytoin fraction.
- In patients with a low plasma albumin
- Phenytoin is approximately 90% protein bound, the free fraction increases, therefore an adjusted total phenytoin should be calculated.
- Disease states that can decrease the albumin concentration and eventually lead to increased free phenytoin concentrations include burns, hepatic cirrhosis, nephrotic syndrome, pregnancy and cystic fibrosis.
Side effects
- Dose-independent
- e.g. hirsutism, acne, coarsening of facial features, gingival hyperplasia, hypocalcaemia and folic acid deficiency
- Concentration-related
- <5 mg/L: generally, no therapeutic effect
- 5-10 mg/L: some anticonvulsant action with approximately 50% of patients obtaining a therapeutic effect with concentrations of 8-10 mg/L
- 10-20 mg/L: optimum concentration for anticonvulsant effect
- 20-30 mg/L: nystagmus, blurred vision
- >30 mg/L: ataxia, dysarthria, drowsiness, coma
Numerous drugs are reported to interact with phenytoin.
Valproic Acid
In view of the lack of a clear concentration-response relationship and the variable pharmacokinetics, there are limited indications for the measurement of valproate levels.
- In most cases, dosage should be based on clinical response.
- In a few cases where seizures are not controlled at high dosage, a plasma level may be helpful in confirming treatment failure.
Valproic acid can take several weeks to become fully active, so adjustment of doses must not be made quickly.
Vancomycin
Vancomycin is a glycopeptide antibiotic commonly used in the treatment of serious gram-positive infections involving S. aureus (MRSA).
- Oral vancomycin is poorly absorbed for systemic use but is indicated for the treatment of Clostridium difficile colitis.
Red man syndrome may occur if the infusion is too rapid.
- It is not an allergic reaction, but may be characterized by hypotension and /or a maculopapular rash appearing on the face, neck, trunk, and/or upper extremities; if this should occur, slow the infusion rate to administer dose over 90 to 120 minutes and increase the dilution volume; the reaction usually dissipates in 30 to 60 minutes; administration of antihistamines just before the infusion may also prevent or minimize this reaction.
- Administer vancomycin with a final concentration not to exceed 5 mg/ml by IV intermittent infusion over at least 60 minutes (recommended infusion period of ≥30 minutes for every 500 mg administered); In adult patients in need of fluid restriction, a concentration up to 10 mg/ml may be used, but risk of infusion-related reactions is increased. Not for IM administration.
Vancomycin is not substantially dialyzed by low-flux haemodialysis membranes made from cellulose acetate or cuprophane, but is by high-flux membranes such as polysulfone, cellulose triacetate and polymethylmethacrylate (30% to 40% over a standard 3- to 4-hr haemodialysis session).
- Vancomycin concentrations in patients with CKD undergoing chronic intermittent haemodialysis will decrease during the dialysis session. The vancomycin concentration will subsequently rise 3-6 hours after cessation of dialysis as vancomycin redistributes into serum from tissue. Thus, it is often recommended that concentrations are drawn 6 hours after dialysis.
- However, such postdialysis monitoring is difficult to coordinate. Predialysis concentrations that are scheduled to be drawn with other laboratory tests may reduce delays in therapy and unnecessary phlebotomy.
Back in 2009, the American Society of Health-System Pharmacists (ASHP), the Infectious Diseases Society of America (IDSA), and the Society of Infectious Diseases Pharmacists (SIDP) published a consensus statement.
- Because it can be difficult in the clinical setting to obtain multiple serum vancomycin concentrations to determine the AUC and subsequently calculate the AUC/MIC, trough serum concentration monitoring, which can be used as a surrogate marker for AUC, is recommended as the most accurate and practical method to monitor vancomycin.
- Based on evidence suggesting that S. aureus exposure to trough serum vancomycin concentrations of less than 10 mg/L can produce strains with VISA-like characteristics, it is recommended that trough serum vancomycin concentrations always be maintained above 10 mg/L to avoid development of resistance.
- Based on the potential to improve penetration, increase the probability of optimal target serum vancomycin concentrations, and improve clinical outcomes for complicated infections such as bacteraemia, endocarditis, osteomyelitis, meningitis, and hospital-acquired pneumonia caused by S. aureus, total trough serum vancomycin concentrations of 15–20 mg/L are recommended. Trough serum vancomycin concentrations in that range should achieve an AUC/MIC of ≥400 in most patients if the MIC is ≤1 mg/L.
In the 2018 article "Making the change to area under the curve-based vancomycin dosing" in American Journal of Health-System Pharmacy, few key points are highlighted.
- Recent data reported by Neely and colleagues support a reassessment of target serum levels for vancomycin, given that aggressive trough concentrations (≥15 mg/L) may not be necessary to achieve desired AUC targets.
- Using a 5,000-patient Monte Carlo simulation, these investigators estimated that approximately 60% of patients could achieve therapeutic AUC values with trough concentrations below 15 mg/L, assuming a vancomycin MIC value of ≤1 mg/L.
- Based on the aforementioned rationale, we recommend a vancomycin AUC of 400–600 mg·hr/L regardless of MIC or organism treated, as the low end represents the threshold for effectiveness for MRSA, the most invasive of pathogens treated with vancomycin, and the upper end represents the apparent upper end of the safety threshold.
- Due to the potential benefits from both a safety and an effectiveness standpoint, an AUC-based dosing approach may be preferable to a trough-based protocol.
Alternatively, you may watch this webinar from Sanford Guide to learn the update.
Monitoring parameter, such as temperature, white blood cell count and culture & sensitivity result.
- Indicates clinical response of the patient.
Toxic effect: Nephrotoxicity and ototoxicity.
- Monitor renal function and audiogram.
Comments
Post a Comment